A major goal in renewable energy research is to harvest the energy of the sun to convert water into hydrogen gas, a storable fuel. Now, with a nanoparticle-based system, researchers have set a record for one of the half-reactions in this process, reporting 100% efficiency for the reduction of water to hydrogen (Nano Lett. 2016, DOI: 10.1021/acs.nanolett.5b04813).
To make such water-splitting systems, researchers must find the right materials to absorb light and catalyze the splitting of water into hydrogen and oxygen. The two half-reactions in this process—the reduction of water to hydrogen gas, and the oxidation of water to oxygen gas—must be isolated from each other so their products don’t react and explode. “Completing the cycle in an efficient, stable, safe fashion with earth-abundant elements is an ongoing challenge,” says chemist Nathan S. Lewis of Caltech, who was not involved in this study.
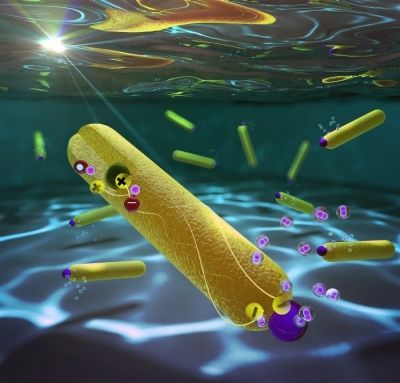
Until recently, the efficiency of the reduction step had maxed out at 60%. One challenge is that electrons and positive charges formed in the light absorption process can rapidly recombine, preventing the electrons from reducing water molecules to form hydrogen. To overcome this problem, several years ago, Lilac Amirav of Technion–Israel Institute of Technology and her colleagues designed a nanoparticle-based system (J. Phys. Chem. Lett. 2010, DOI: 10.1021/jz100075c) that would physically separate the charges formed during photocatalysis.