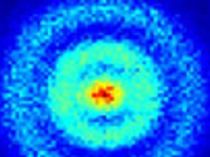
What you’re looking at is the first direct observation of an atom’s electron orbital — an atom’s actual wave function! To capture the image, researchers utilized a new quantum microscope — an incredible new device that literally allows scientists to gaze into the quantum realm.
An orbital structure is the space in an atom that’s occupied by an electron. But when describing these super-microscopic properties of matter, scientists have had to rely on wave functions — a mathematical way of describing the fuzzy quantum states of particles, namely how they behave in both space and time. Typically, quantum physicists use formulas like the Schrödinger equation to describe these states, often coming up with complex numbers and fancy graphs.
Up until this point, scientists have never been able to actually observe the wave function. Trying to catch a glimpse of an atom’s exact position or the momentum of its lone electron has been like trying to catch a swarm of flies with one hand; direct observations have this nasty way of disrupting quantum coherence. What’s been required to capture a full quantum state is a tool that can statistically average many measurements over time.