This publication suggests that wax could be carried on vehicles and used to create hydrogen gas in situ, the waste carbon being used to make more wax via syngas production and the Fischer-Tropsch process, where carbon monoxide and hydrogen is converted into hydrocarbons as a potential source of petro-chemicals that does not involve releasing fossil carbon into the atmosphere. While this publication is still a long way from a working industrial-scale process, it offers a very hopeful potential avenue for less-polluting technology.
Philip recently attended an event for other Oxford University chemistry alumni, and one of the speakers drew attention to a recent publication from, among others, Oxford chemists, regarding the production of hydrogen from paraffin waxes by microwave degradation using a ruthenium catalyst.
Hydrogen has often been suggested as an environmentally-friendly replacement energy source for fossil fuels in transport vehicles and other applications requiring high energy density. (Note that hydrogen is not a “fuel”, as it must be made using energy from other sources, which can be environmentally-friendly or not.) However, there are significant problems with this, notably involving the safe storage of a highly-inflammable and explosive gas which is much lighter than air.
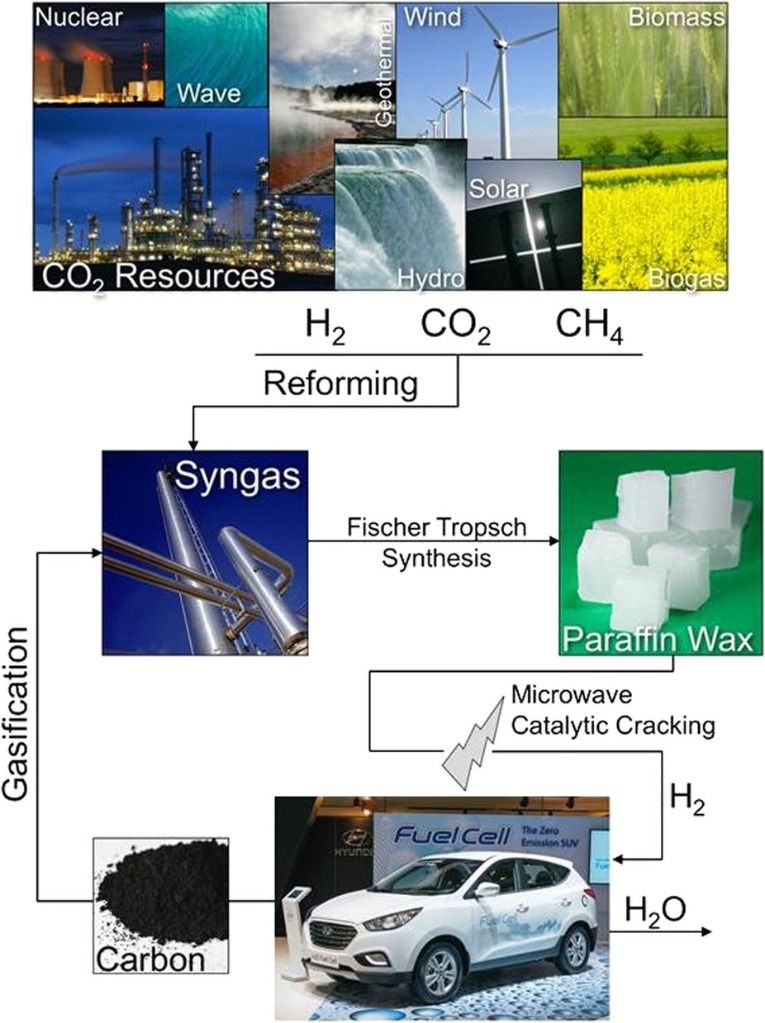
Th idea of a car, truck or ship getting its hydrogen in situ from wax is fascinating as it solves the hydrogen compression/storage problem — with “the waste carbon being used to make more wax.” Too bad this is “still a long way from a working industrial-scale process.“
In the meantime there’s an industrial scale process to make hydrogen that’s ready to go right now — with a 78% conversion rate. And the only byproduct is power carbon, useful in itself:
“Fine methane bubbles are injected at the bottom of a column filled with molten tin. The cracking reaction happens when these bubbles rise to the surface of the liquid metal. Carbon separates on the surface of the bubbles and is deposited as a powder at the top end of the reactor … clogging is avoided because the microgranular carbon powder produced can be easily separated. The reactor thus guarantees the technical preconditions that would be needed for the continuous operation of an industrial-scale reactor.”
http://www.kit.edu/kit/english/pi_2015_139_crack-it-energy-from-a-fossil-fuel-without-carbon-di-oxide.php