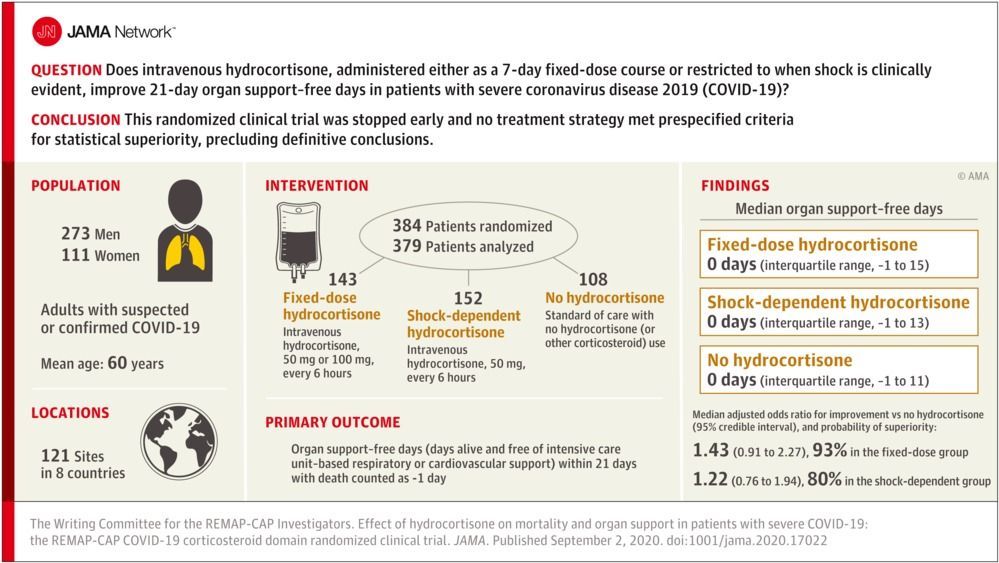
Findings In this bayesian randomized clinical trial that included 403 patients and was stopped early after results from another trial were released, treatment with a 7-day fixed-dose course of hydrocortisone or shock-dependent dosing of hydrocortisone, compared with no hydrocortisone, resulted in 93% and 80% probabilities of superiority, respectively, with regard to the odds of improvement in organ support–free days within 21 days.
Objective To determine whether hydrocortisone improves outcome for patients with severe COVID-19.
Design, Setting, and Participants An ongoing adaptive platform trial testing multiple interventions within multiple therapeutic domains, for example, antiviral agents, corticosteroids, or immunoglobulin. Between March 9 and June 17, 2020, 614 adult patients with suspected or confirmed COVID-19 were enrolled and randomized within at least 1 domain following admission to an intensive care unit (ICU) for respiratory or cardiovascular organ support at 121 sites in 8 countries. Of these, 403 were randomized to open-label interventions within the corticosteroid domain. The domain was halted after results from another trial were released. Follow-up ended August 12, 2020.
Interventions The corticosteroid domain randomized participants to a fixed 7-day course of intravenous hydrocortisone (50 mg or 100 mg every 6 hours) (n = 143), a shock-dependent course (50 mg every 6 hours when shock was clinically evident) (n = 152), or no hydrocortisone (n = 108).