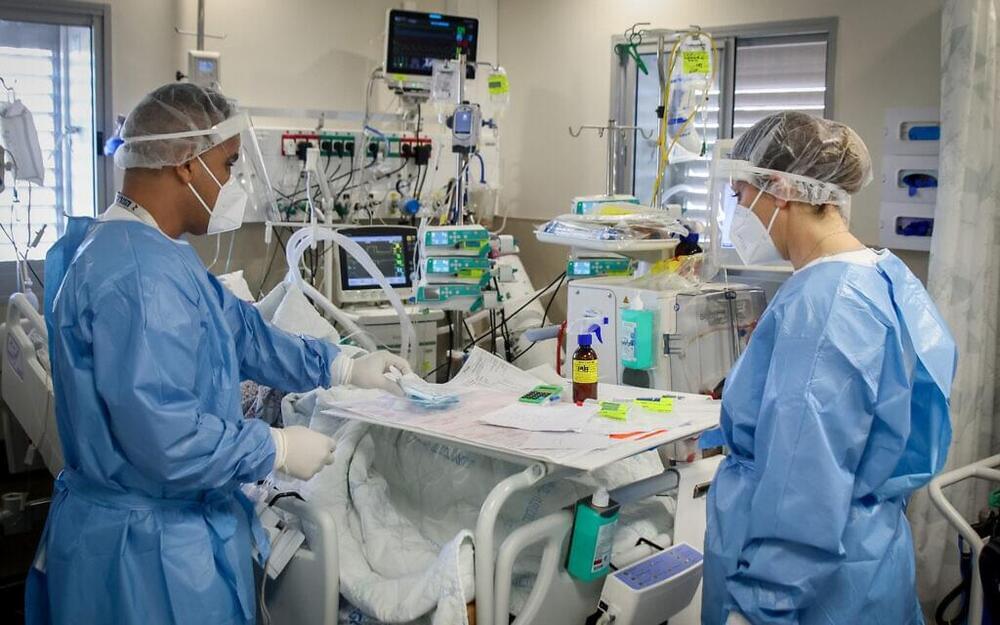
Safed’s Ziv Medical Center has sent a letter to Health Ministry director Nachman Ash requesting he grant emergency authorization for the anti-COVID drug Amor 18 developed by the Israeli company Amorphical in order to treat patients in moderate to serious condition due to the coronavirus, Channel 2 reported Friday.
In letter to Health Ministry director, Ziv Medical Center points to promising results from Amor 18’s clinical trial, where all patients who received drug went on to recover (drug uses Amorphous Calcium Carbonate: ACC).
Israeli biotech company Amorphical recently published what it says are promising results from the second stage of its Amor 18 clinical study.
-please note: Regeneron helped end the ebola pandemic by ending clinical trials early and getting treatments to patients ASAP.
Clinical trial: https://clinicaltrials.gov/ct2/show/NCT04900337