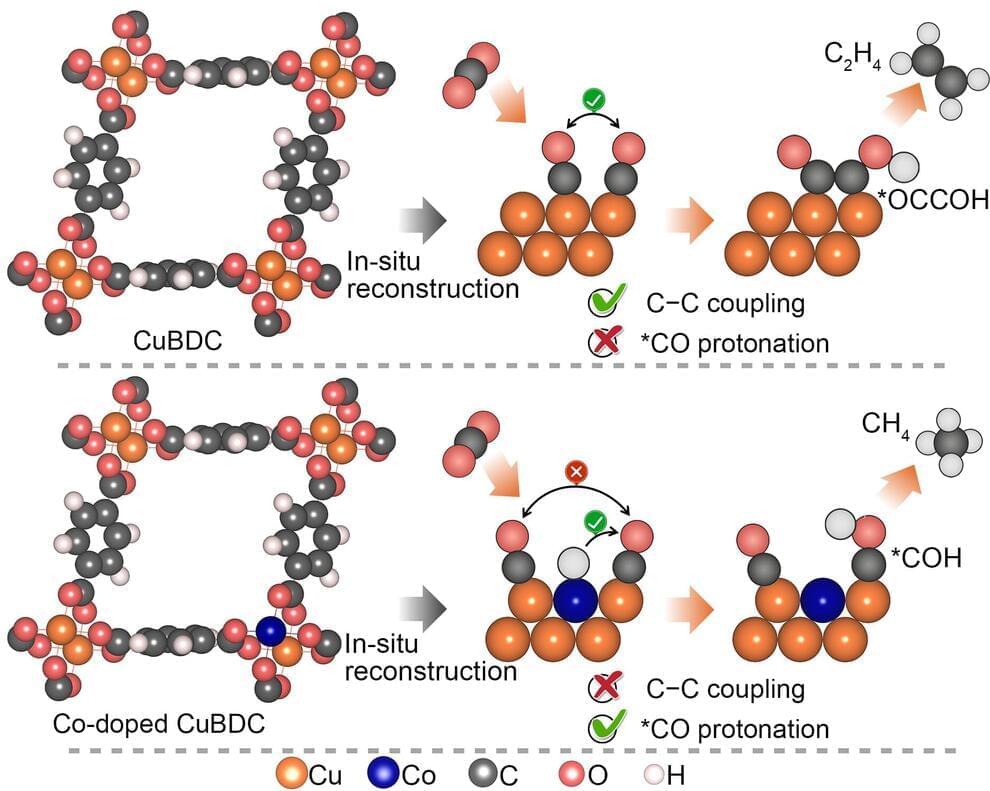
The world is highly dependent on fossil fuels to power its industry and transportation. These fossil fuels lead to excessive carbon dioxide emission, which contributes to global warming and ocean acidification. One way to reduce this excessive carbon dioxide emission that is harmful to the environment is through the electroreduction of carbon dioxide into value-added fuels or chemicals using renewable energy. The idea of using this technology to produce methane has attracted wide interest. However, researchers have had limited success in developing efficient catalysts for methane.
A Soochow University research team has now developed a simple strategy for creating cobalt copper alloy catalysts that deliver outstanding methane activity and selectivity in electrocatalytic carbon dioxide reduction. Their research is published in Nano Research.
Over the past 10 years, scientists have made notable progress in advancing their understanding of catalysts and applying the knowledge to their fabrication. But the catalysts that have been developed have not been satisfactory for use with methane, in terms of selectivity or current density. Despite the great insights scientists have gained, the strategies they have attempted in creating catalysts for methane are just too costly to be useful in practical applications.