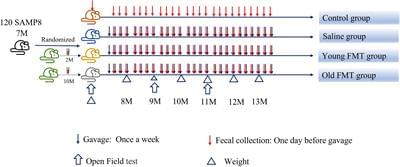
Recent evidence points out the role of the gut microbiota in the aging process. However, the specific changes and relevant interventions remain unclear. In this study, Senescence Accelerated Mouse-Prone 8 (SAMP8) mice were divided into four groups; young-FMT-group transplanted fecal microbiota from young donors (2–3°months old) and old-FMT-group transplanted from old donors (10–11°months old); additionally, other two groups either adult mice injected with saline solution or untreated mice served as the saline and blank control groups, respectively. All mice were intervened from their 7-months-old until 13-months-old. The open field test at 9 and 11°months of age showed that the mice transplanted with gut microbiota from young donors had significantly better locomotor and exploration ability than those of transplanted with old-donors gut microbiota and those of saline control while was comparable with the blank control. 16S rRNA gene sequencing showed that the gut microbiome of recipient mice of young donors was altered at 11°months of age, whereas the alternation of the gut microbiome of old-donor recipient mice was at 9°months. For comparison, the recipient mice in the blank and saline control groups exhibited changes in the gut microbiome at 10°months of age. The hallmark of aging-related gut microbiome change was an increase in the relative abundance of Akkermansia, which was significantly higher in the recipients transplanted with feces from older donors than younger donors at 9°months of age. This study shows that fecal microbiota transplantation from younger donors can delay aging-related declines in locomotor and exploration ability in mice by changing the gut microbiome.
Aging is inherently accompanied by the decline of physical and mental abilities, including locomotor, cognition, and bodily functions, to subsequently cause frailty syndrome, neurodegenerative diseases, and other age-related diseases, which reduce the quality of life of the aging population (Hou et al., 2019). Aging mechanisms and anti-aging interventions have long been a major focus of biomedical research, which is particularly relevant given the rapidly aging society.
The gut is a major organ for nutrients absorption, metabolism, and immunity, and contains hundreds of millions of microorganisms and their metabolites, which comprise the gut microbiota (Heintz and Mair, 2014) that interacts with host cells and tissues (Huang et al., 2021). Our previous study reported continuous changes in the gut microbiome of centenarians during their transition from a healthy status to death. The most significant changes of gut microbial communities in the period were found to occur at 7°months prior to death, suggesting that this may be a turning point of significant changes in the gut microbiome of centenarians (Luan et al., 2020). Recent studies have revealed an important relationship between the gut microbiome and aging-related diseases such as Alzheimer disease (Ticinesi et al., 2018; Haran and McCormick, 2021), suggesting that the gut microbiome plays an essential role in the aging process.