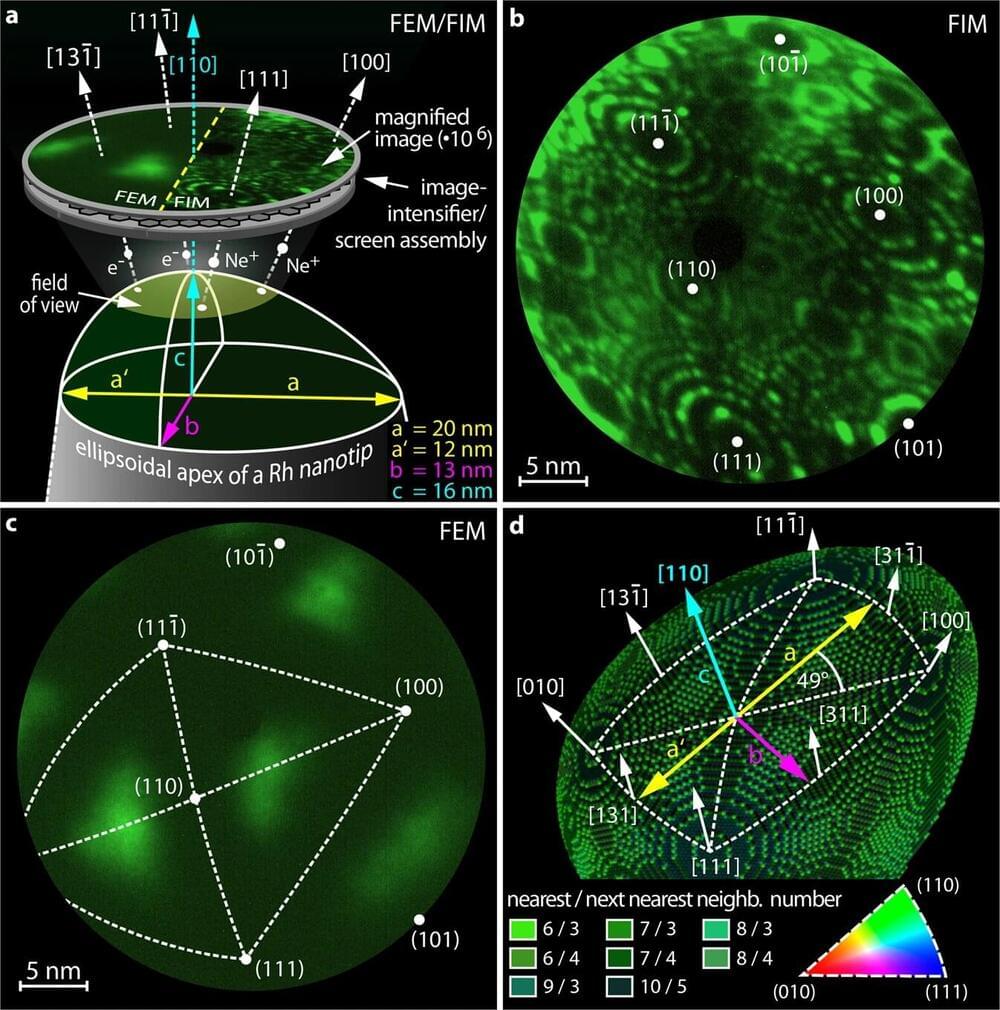
Chaotic behavior is typically known from large systems: for example, from weather, from asteroids in space that are simultaneously attracted by several large celestial bodies, or from swinging pendulums that are coupled together. On the atomic scale, however, one does normally not encounter chaos—other effects predominate.
Now, for the first time, scientists at TU Wien have been able to detect clear indications of chaos on the nanometer scale—in chemical reactions on tiny rhodium crystals. The results have been published in the journal Nature Communications.
The chemical reaction studied is actually quite simple: with the help of a precious metal catalyst, oxygen reacts with hydrogen to form water, which is also the basic principle of a fuel cell. The reaction rate depends on external conditions (pressure, temperature). Under certain conditions, however, this reaction shows oscillating behavior, even though the external conditions are constant.