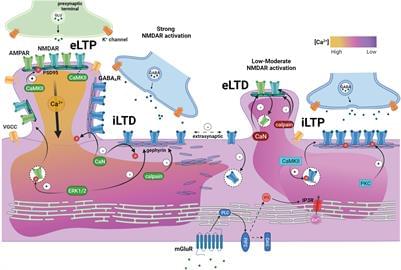
Synaptic plasticity is a critical process that regulates neuronal activity by allowing neurons to adjust their synaptic strength in response to changes in activity. Despite the high proximity of excitatory glutamatergic and inhibitory GABAergic postsynaptic zones and their functional integration within dendritic regions, concurrent plasticity has historically been underassessed. Growing evidence for pathological disruptions in the excitation and inhibition (E/I) balance in neurological and neurodevelopmental disorders indicates the need for an improved, more “holistic” understanding of synaptic interplay. There continues to be a long-standing focus on the persistent strengthening of excitation (excitatory long-term potentiation; eLTP) and its role in learning and memory, although the importance of inhibitory long-term potentiation (iLTP) and depression (iLTD) has become increasingly apparent. Emerging evidence further points to a dynamic dialogue between excitatory and inhibitory synapses, but much remains to be understood regarding the mechanisms and extent of this exchange. In this mini-review, we explore the role calcium signaling and synaptic crosstalk play in regulating postsynaptic plasticity and neuronal excitability. We examine current knowledge on GABAergic and glutamatergic synapse responses to perturbances in activity, with a focus on postsynaptic plasticity induced by short-term pharmacological treatments which act to either enhance or reduce neuronal excitability via ionotropic receptor regulation in neuronal culture. To delve deeper into potential mechanisms of synaptic crosstalk, we discuss the influence of synaptic activity on key regulatory proteins, including kinases, phosphatases, and synaptic structural/scaffolding proteins. Finally, we briefly suggest avenues for future research to better understand the crosstalk between glutamatergic and GABAergic synapses.
Ligand-gated ion channel GABA type A receptors (GABAARs) mediate the majority of fast inhibition in the central nervous system, while glutamatergic AMPA receptors (AMPARs) and NMDA receptors (NMDARs) collectively mediate fast excitatory neurotransmission. NMDARs particularly play a unique role in synaptic plasticity due to high calcium permeability and voltage-dependent Mg2+ block typically relieved by AMPAR-mediated depolarization. Slow inhibition and excitation are generated by G protein-coupled, GABA type B (GABABRs) and metabotropic glutamate receptors (mGluRs), respectively. The concerted action of these receptors balances neuronal excitability. A close and coordinated spatial relationship between glutamatergic and GABAergic synapses on dendrites (Megías et al., 2001; Bleckert et al., 2013; Iascone et al., 2020), sometimes as near as on the same spine (Chen et al., 2012), facilitates synaptic input integration, dynamic calcium regulation, synaptic crosstalk, and coregulation.
Synaptic plasticity describes the ability of synapses to adapt their relative strength based on the overall level of activity or specific activity patterns, often by dynamic regulation of receptor-synaptic scaffold interactions or through trafficking. During development, it is heavily involved in dendritic growth, synaptogenesis, and the formation of neural circuits (reviewed in Akgül and McBain, 2016; Ismail et al., 2017; Jenks et al., 2021). In mature neurons, synaptic plasticity is responsible for synapse remodeling during experience. Genetic mutations or pathology leading to altered excitatory or inhibitory neurotransmission or impaired synaptogenesis typically result in deficits in synaptic plasticity, a common feature in neurodevelopmental and neurological disorders (Rudolph and Möhler, 2014; Mele et al., 2019), including autism (Hansel, 2019; Sohal and Rubenstein, 2019), down syndrome (Galdzicki et al., 2001; Schulz et al.