Antibiotic resistance is a major danger to public health that threatens to claim the lives of millions of people per year within the next few decades. Years of necessary administration and excessive application of antibiotics have selected for strains that are resistant to many of our currently available treatments. Due to the high costs and difficulty of developing new antibiotics, the emergence of resistant bacteria is outpacing the introduction of new drugs to fight them. To overcome this problem, many researchers are focusing on developing antibacterial therapeutic strategies that are “resistance-resistant”—regimens that slow or stall resistance development in the targeted pathogens. In this mini review, we outline major examples of novel resistance-resistant therapeutic strategies. We discuss the use of compounds that reduce mutagenesis and thereby decrease the likelihood of resistance emergence. Then, we examine the effectiveness of antibiotic cycling and evolutionary steering, in which a bacterial population is forced by one antibiotic toward susceptibility to another antibiotic. We also consider combination therapies that aim to sabotage defensive mechanisms and eliminate potentially resistant pathogens by combining two antibiotics or combining an antibiotic with other therapeutics, such as antibodies or phages. Finally, we highlight promising future directions in this field, including the potential of applying machine learning and personalized medicine to fight antibiotic resistance emergence and out-maneuver adaptive pathogens.
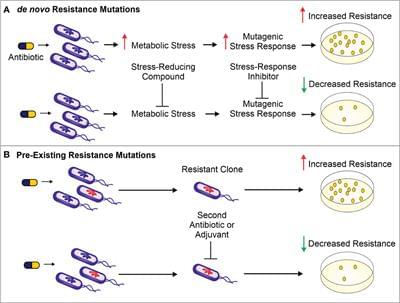
The use of antibiotics is central to the practice of modern medicine but is threatened by widespread antibiotic resistance (Centers for Disease Control and Prevention (U.S.), 2019). Antibiotics are a selective evolutionary pressure—they inhibit bacterial growth and viability, and antibiotic-treated bacteria are forced to either adapt and survive or succumb to treatment. The stress of antibiotic treatment can enhance bacterial mutagenesis leading to de novo resistance mutations (Figure 1A), promote the acquisition of horizontally transferred genetic elements that confer resistance, or trigger phenotypic responses that increase tolerance to drugs (Davies and Davies, 2010; Levin-Reisman et al., 2017; Bakkeren et al., 2019; Darby et al., 2022;). Additionally, antibiotic treatment can select for the proliferation of pre-existing mutants already in the population (Figure 1B).