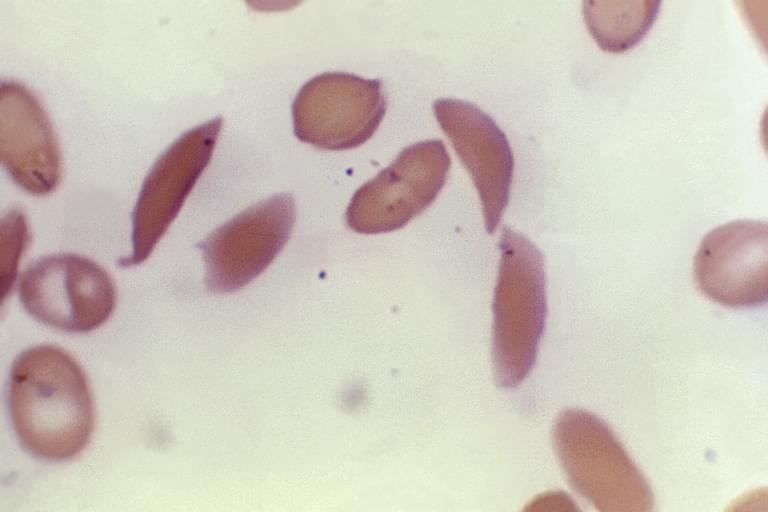
LONDON (AP) — Britain’s medicines regulator has authorized the world’s first gene therapy treatment for sickle cell disease, in a move that could offer relief to thousands of people with the crippling disease in the U.K. In a statement Thursday, the Medicines and Healthcare Regulatory Agency said it approved Casgevy, the first medicine licensed using the gene editing tool CRISPR, which won its makers a Nobel prize in 2020. The agency approved…