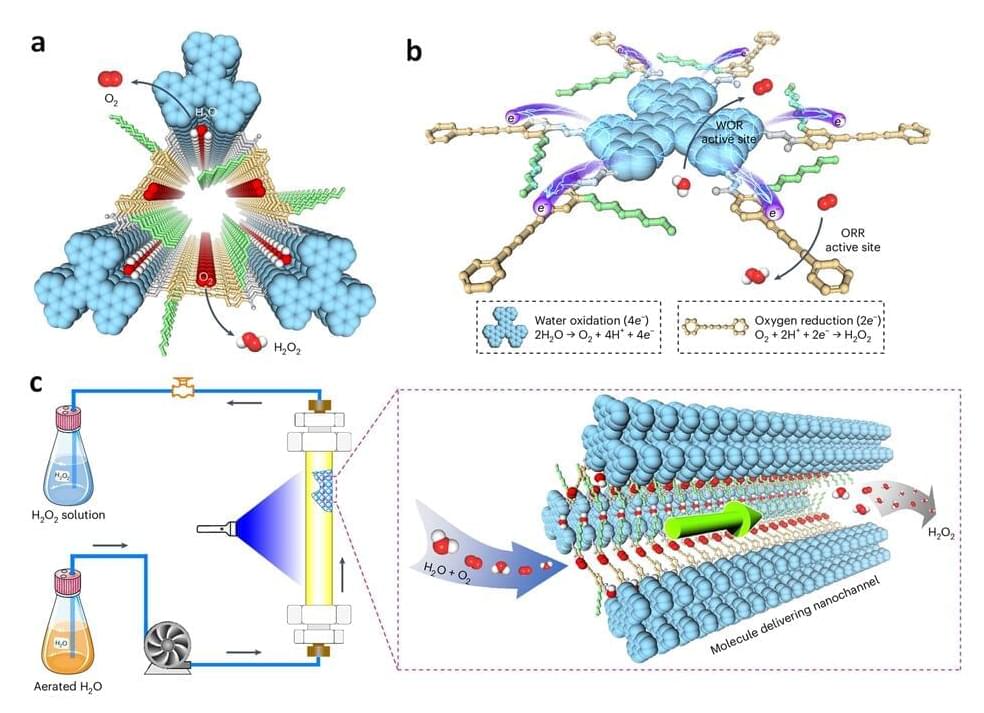
National University of Singapore (NUS) chemists have developed hexavalent photocatalytic covalent organic frameworks (COFs) which mimic natural photosynthesis for the production of hydrogen peroxide (H 2 O 2), an important industrial chemical.
The conventional method of H 2 O 2 production involves using anthraquinone as a catalyst to convert air and hydrogen into H 2 O 2. However, this process requires substantial energy, costly noble metal catalysts, high-pressure hydrogen gas and hazardous solvents. Artificial photosynthesis of H 2 O 2, resembling the natural photosynthesis process with the use of sunlight as an energy source and abundant water and air as feedstocks, presents a sustainable and promising alternative to the conventional anthraquinone process.
However, such an artificial system faces three key challenges: insufficient charge carrier generation and fast charge recombination, which lowers the efficiency; limited number of available catalytic sites, which results in low productivity; and lack of efficient delivery of charges and reactants to the catalytic sites, which causes sluggish reaction kinetics.