Researchers have developed a novel method using facet-selective, ultrafine cocatalysts to efficiently split water to create hydrogen – a clean source of fuel. Scientists are urgently searching for clean fuel sources – such as hydrogen – to move towards carbon neutrality. A breakthrough for improving the efficiency of the photocatalytic reaction that splits water into hydrogen has been made by a team of researchers from Tohoku University, Tokyo University of Science and Mitsubishi Materials Corporation.
“Water-splitting photocatalysts can produce hydrogen (H2) from only sunlight and water,” explains Professor Yuichi Negishi, the lead researcher of this project (Tohoku University), “However, the process hasn’t been optimized sufficiently for practical applications. If we can improve the activity, hydrogen can be harnessed for the realization of a next-generation energy society.”
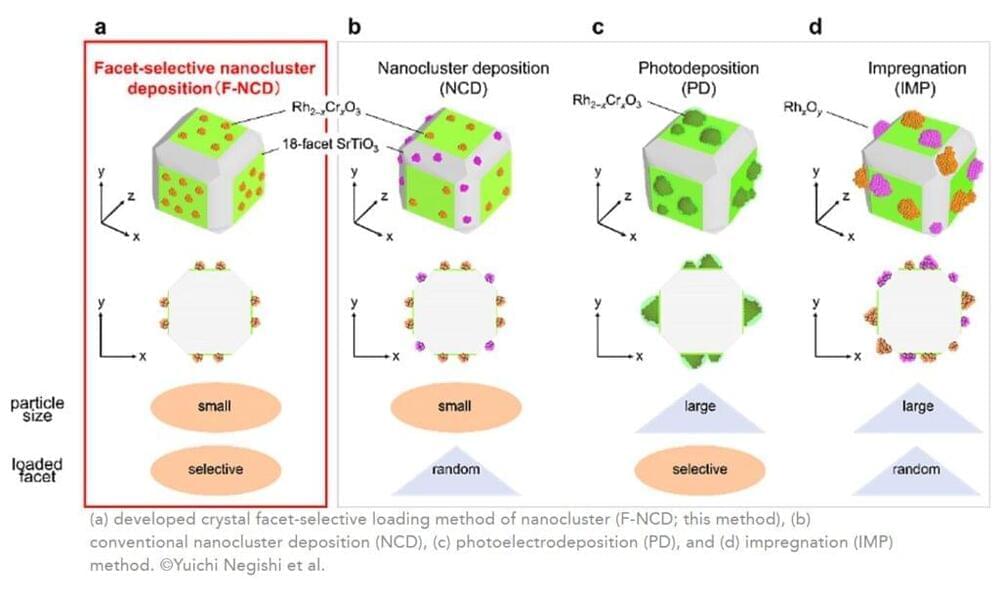
The research team established a novel method that uses ultrafine rhodium (Rh)-chromium (Cr) mixed-oxide (Rh2-xCrxO3) cocatalysts (the actual reaction site and a key component to stop H2 reforming with oxygen to make water again) with a particle size of about 1 nm. Then, they are loaded crystal facet-selectively onto a photocatalyst (uses sunlight and water to speed up reactions). Previous studies have not been able to accomplish these two feats in a single reaction: a tiny cocatalyst that can also be placed on specific regions of the photocatalyst.









Leave a reply