Biomedical engineers at Duke University have shown that a genetic mutation that causes congenital heart disease also contributes to kidney damage and developmental defects. Identifying this early cause of kidney damage could enable clinicians to diagnose and address kidney problems much sooner than current practices allow. The research was published on November 3 in the journal Nature Biomedical Engineering.
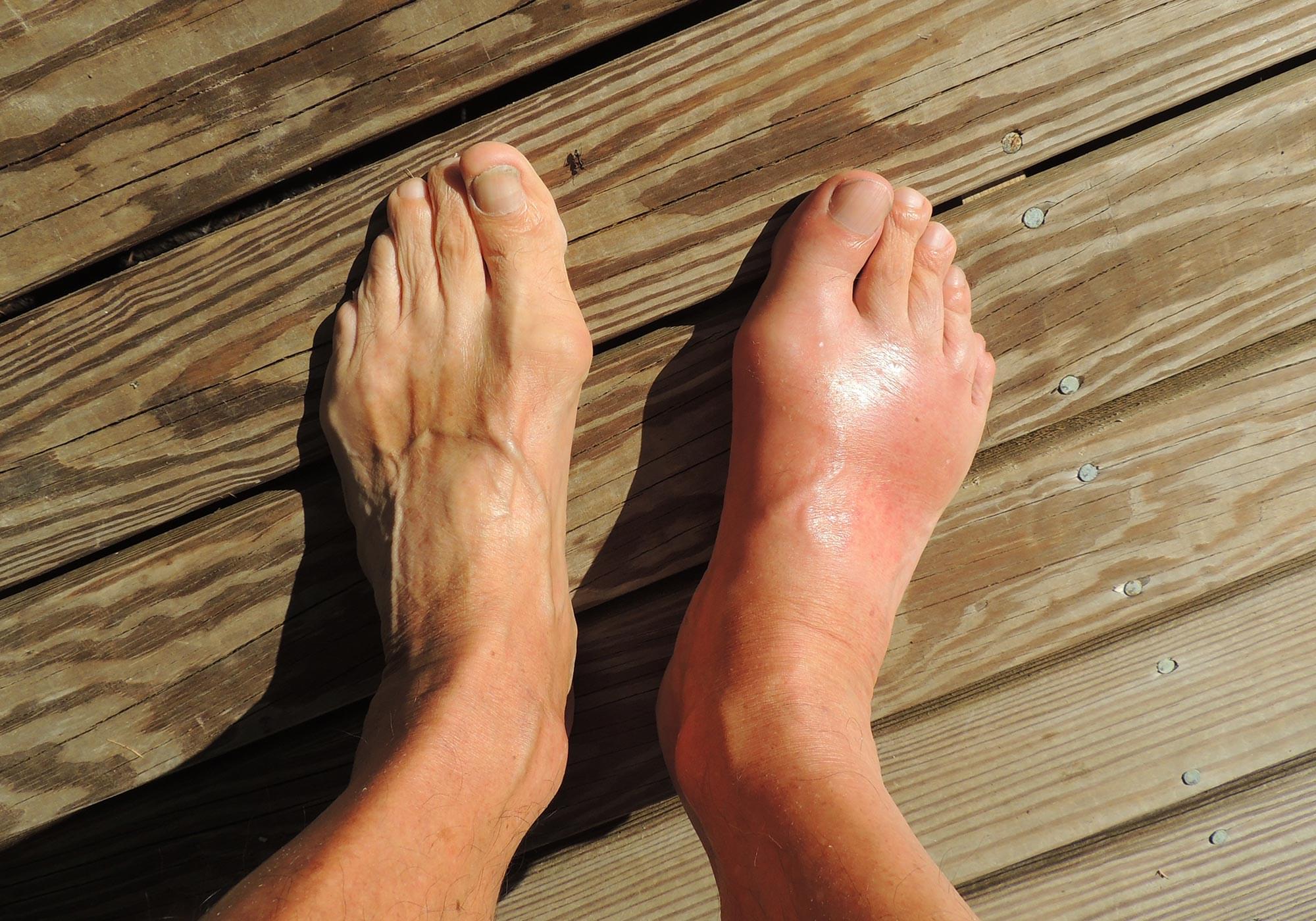
Congenital heart disease (CHD) is a common cause of death in childhood and affects 1 out of every 1,000 births. The disease occurs when the heart doesn’t form correctly before birth, causing leaky valves, defective vessels, or holes in the heart. While some cases of CHD can be remedied, children with life-threatening complications often require surgery or even a heart transplant. More than 25% of patients also end up developing problems with other organs, which severely compromise life expectancy.
“Research has shown that children diagnosed with CHD almost always have kidney problems by age 4,” said Samira Musah, the Alfred M. Hunt Faculty Scholar Assistant Professor of Biomedical Engineering and Assistant Professor of Medicine at Duke University, and the senior author of the study. “Given the shared developmental origin of the heart and kidney, I wondered if a genetic mutation tied to CHD also causes the kidney damage observed in affected patients.”