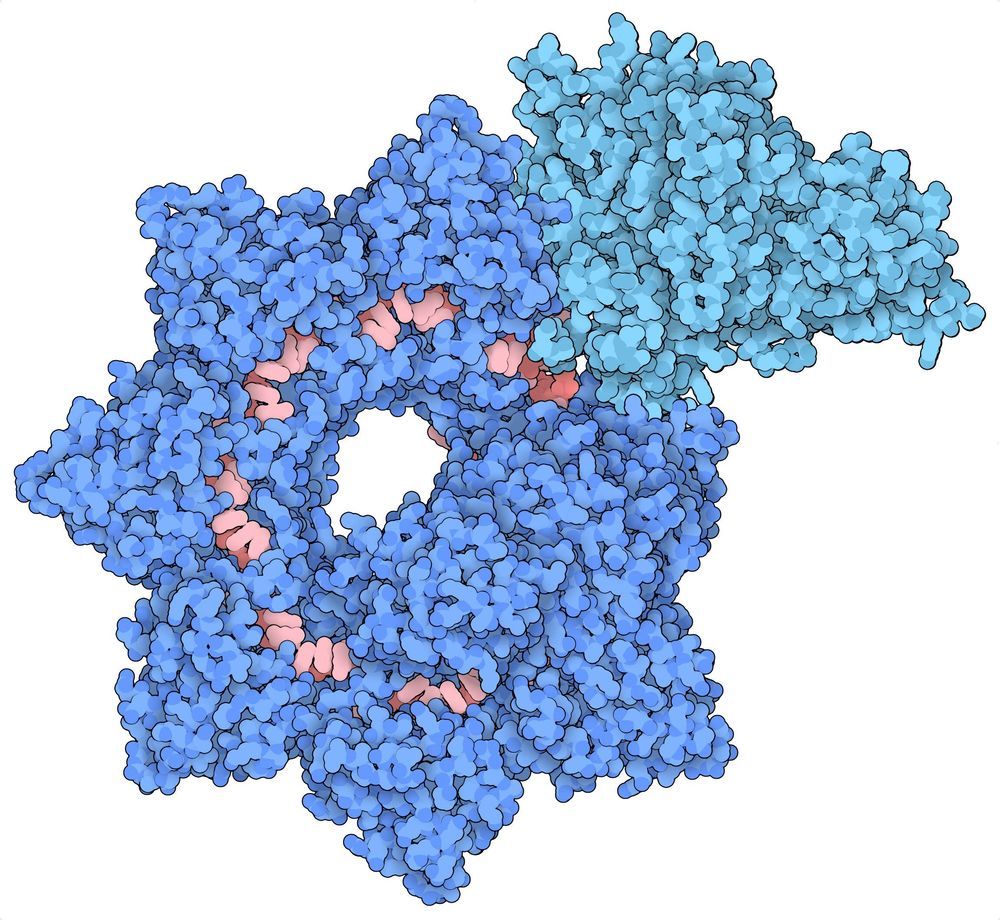
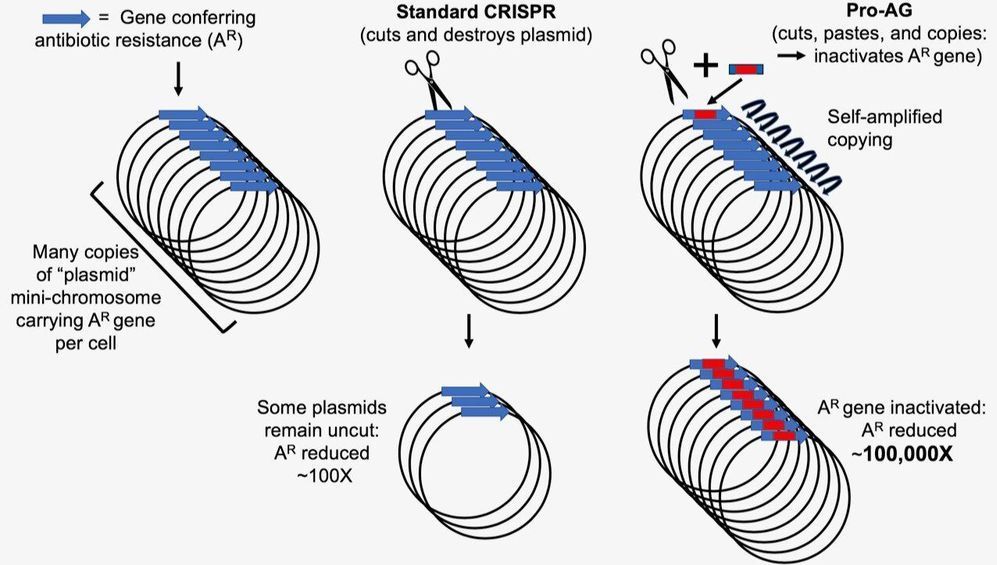
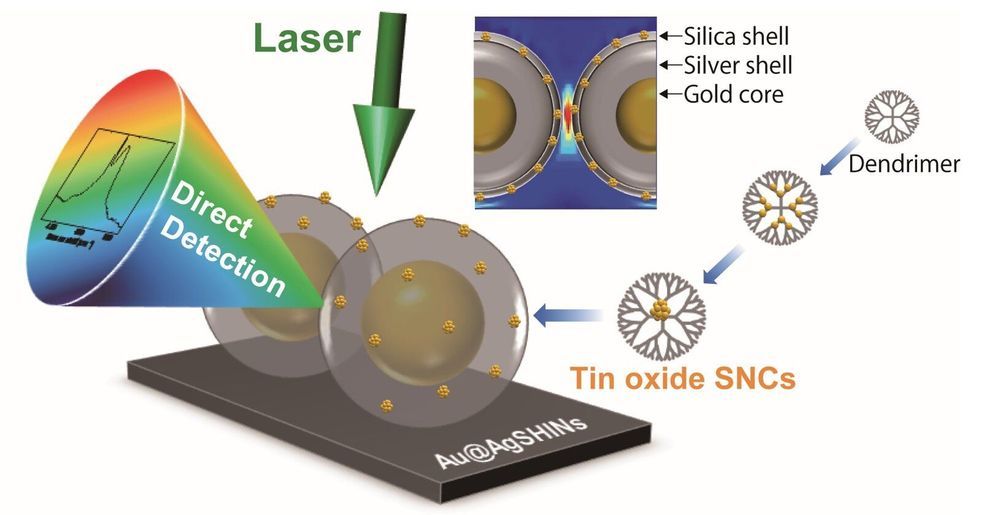
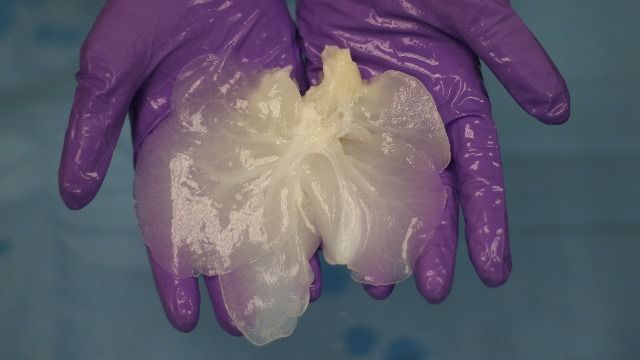
With new technology to edit genes, scientists are now working on things that once seemed impossible. But what are the boundaries? See the full 60 Minutes interview with Church, here: https://cbsn.ws/34ZhuTs
Watch Full Episodes of “60 Minutes” HERE: http://cbsn.ws/1Qkjo1F
Get more “60 Minutes” from “60 Minutes: Overtime” HERE: http://cbsn.ws/1KG3sdr
Relive past episodies and interviews with “60 Rewind” HERE: http://cbsn.ws/1PlZiGI
Follow “60 Minutes” on Instagram HERE: http://bit.ly/23Xv8Ry
Like “60 Minutes” on Facebook HERE: http://on.fb.me/1Xb1Dao
Follow “60 Minutes” on Twitter HERE: http://bit.ly/1KxUsqX
Follow “60 Minutes” on Google+ HERE: http://bit.ly/1KxUvmG
Get unlimited ad-free viewing of the latest stories plus access to classic 60 Minutes archives, 60 Overtime, and exclusive extras. Subscribe to 60 Minutes All Access HERE: http://cbsn.ws/23XvRSS
Get the latest news and best in original reporting from CBS News delivered to your inbox. Subscribe to newsletters HERE: http://cbsn.ws/1RqHw7T
Get your news on the go! Download CBS News mobile apps HERE: http://cbsn.ws/1Xb1WC8
Get new episodes of shows you love across devices the next day, stream local news live, and watch full seasons of CBS fan favorites anytime, anywhere with CBS All Access. Try it free! http://bit.ly/1OQA29B