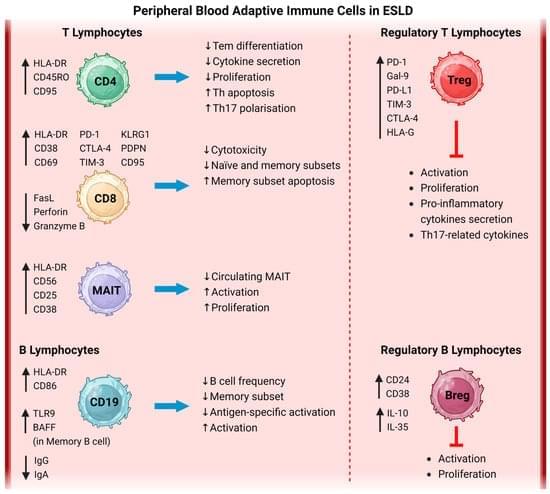
The incidence and prevalence of cutaneous melanoma in the US and worldwide have increased over the last 5 decades.
Cutaneous melanoma presents as a new, changing, or irregularly pigmented skin lesion. Risk factors for cutaneous melanoma include UV radiation exposure, skin type, presence of benign and atypical nevi, and personal or family history of melanoma.
This Review summarizes current evidence regarding the epidemiology, pathophysiology, diagnosis, and treatment of cutaneous melanoma.
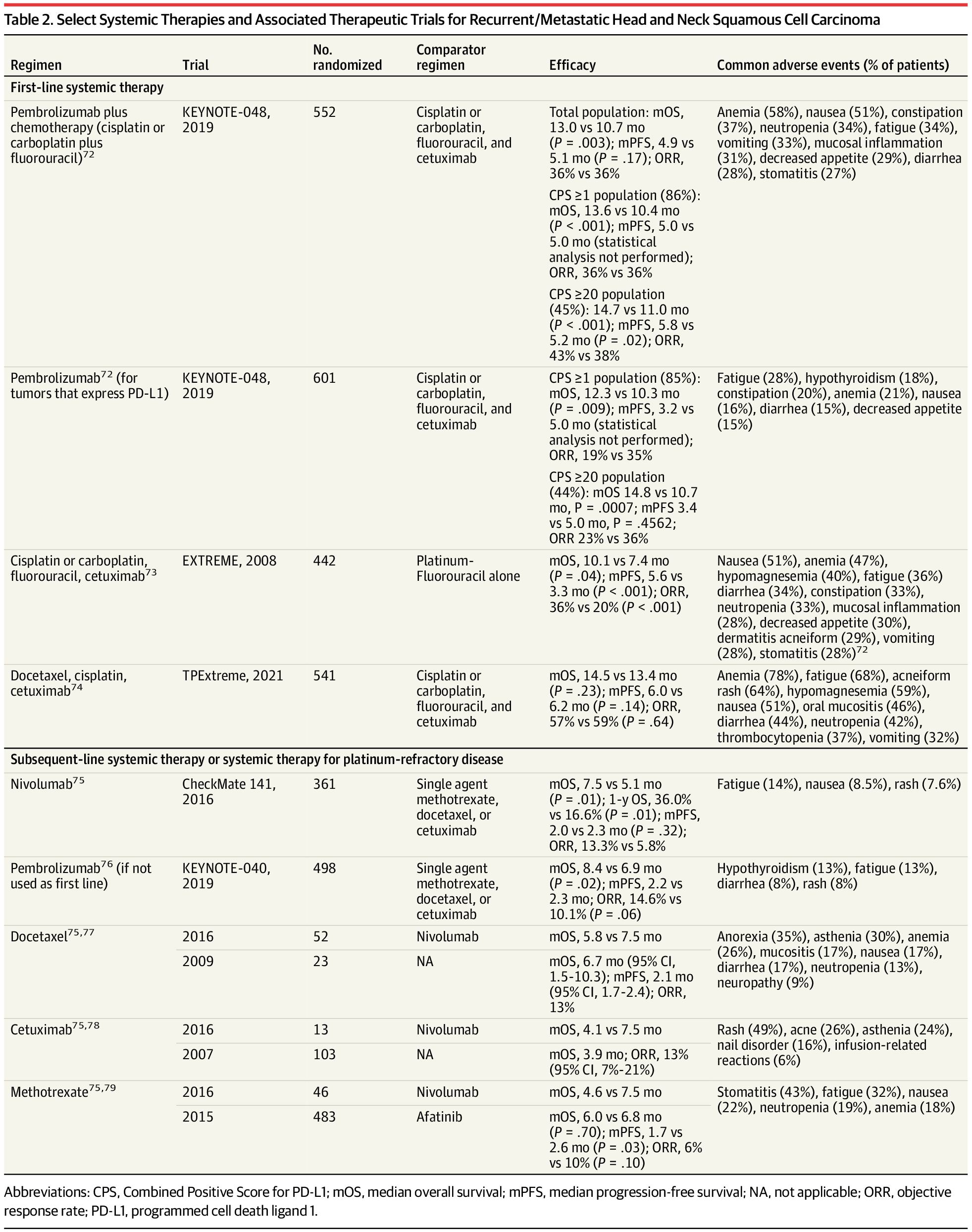
Improvements in melanoma mortality over the last decade are attributed to the advent of multiple effective therapies,6 including immune checkpoint blockade with anti–cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) antibodies (ipilimumab), anti–programmed cell death protein 1 (PD-1) antibodies (nivolumab, pembrolizumab), and anti–lymphocyte activation gene 3 protein (LAG-3) antibodies (relatlimab), as well as oral combination targeted therapy with B-Raf protein (BRAF) and mitogen-activated extracellular signal-regulated kinase (MEK) inhibitors (eg, encorafenib + binimetinib, vemurafenib + cobimetinib, dabrafenib + trametinib).
This review summarizes current evidence regarding epidemiology and risk factors, clinical presentation, diagnosis, and management of cutaneous melanoma (Box).