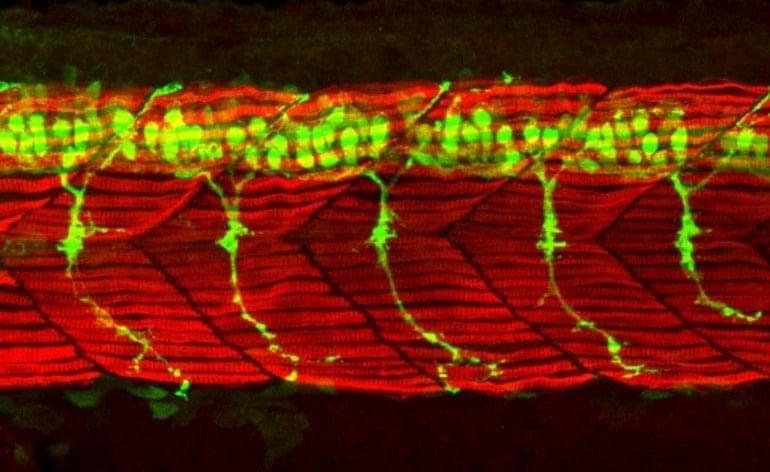
The liver’s ability to regenerate itself is legendary. Even if more than 70% of the organ is removed, the remaining tissue can regrow an entire new liver.
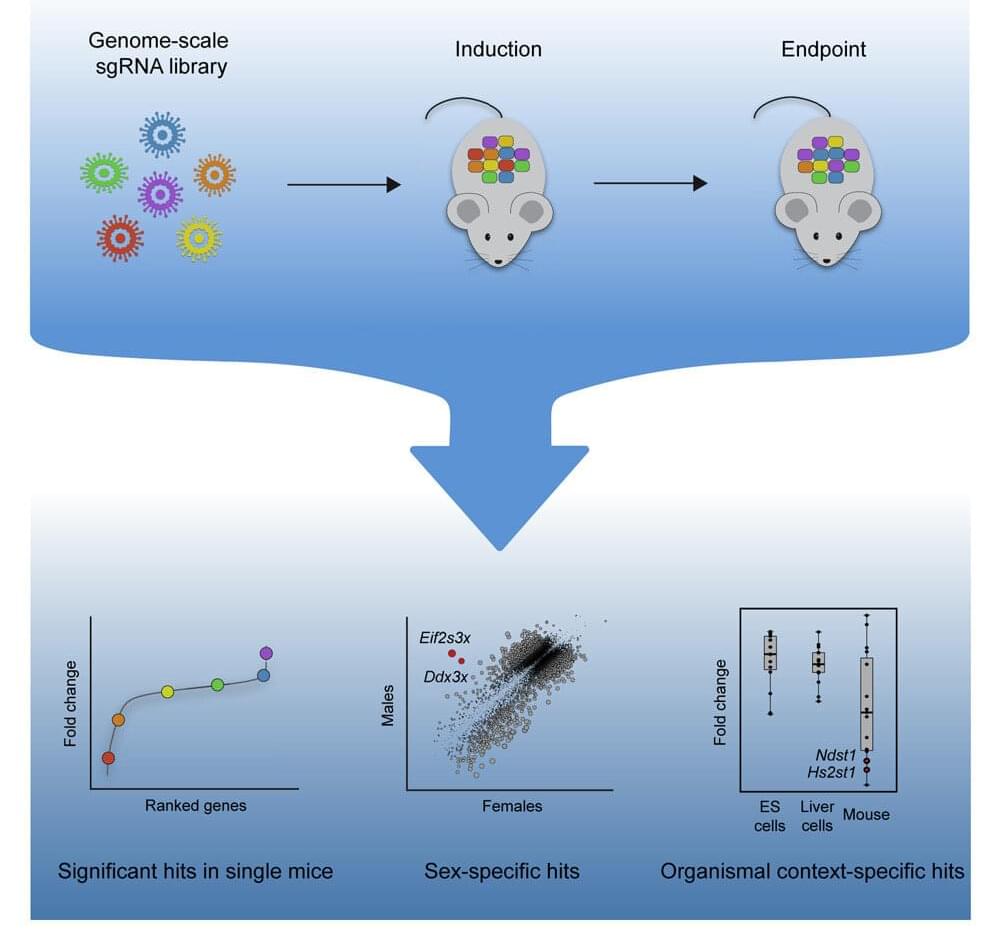
Kristin Knouse, an MIT assistant professor of biology, wants to find out how the liver is able to achieve this kind of regeneration, in hopes of learning how to induce other organs to do the same thing. To that end, her lab has developed a new way to perform genome-wide studies of the liver in mice, using the gene-editing system CRISPR.
With this new technique, researchers can study how each of the genes in the mouse genome affects a particular disease or behavior. In a paper describing the technique, the researchers uncovered several genes important for liver cell survival and proliferation that had not been seen before in studies of cells grown in a lab dish.