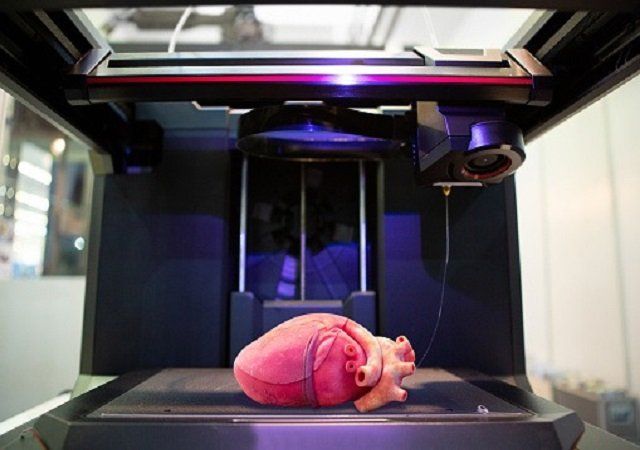
“One of the biggest roadblocks to generating functional tissue replacements has been our inability to print the complex vasculature that can supply nutrients to densely populated tissues,” said Jordan Miller, assistant professor at Rice University in the US.
“Further, our organs actually contain independent vascular networks — like the airways and blood vessels of the lung or the bile ducts and blood vessels in the liver,” Miller said.
“These interpenetrating networks are physically and biochemically entangled, and the architecture itself is intimately related to tissue function. Ours is the first bioprinting technology that addresses the challenge of multi vascularisation in a direct and comprehensive way,” he said.