A rare dual heart and liver transplant gave an 11-year-old girl a new chance at life.


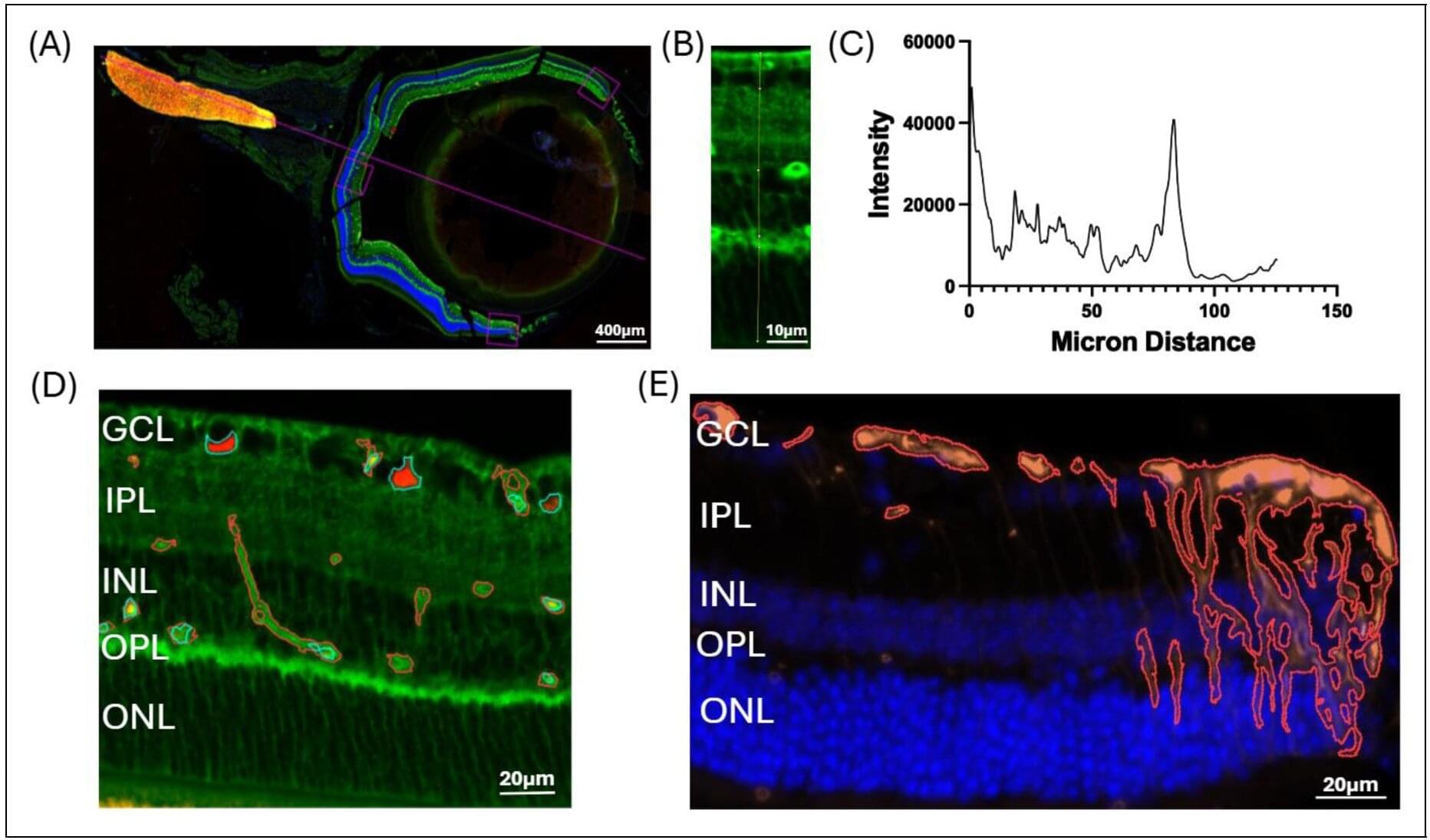
The eyes—specifically, the outer area of the retina—may provide a window into early detection of Alzheimer’s disease (AD) long before irreversible brain damage has occurred, according to new research from Houston Methodist. This discovery could dramatically change how the disease is diagnosed, monitored and treated.
“Retinal Müller glia alterations and their impact on ocular glymphatic clearance in an Alzheimer’s disease mouse model,” is online and will appear in an upcoming edition of the Journal of Alzheimer’s Disease. Led by Stephen Wong, Ph.D., the John S. Dunn Presidential Distinguished Chair in Biomedical Engineering at Houston Methodist and director of T. T. & W. F. Chao Center for BRAIN, the study reveals how the peripheral retina (versus the central retina) could be a window into early diagnosis of AD.
“The eyes are indeed a window into the brain, but our study reveals that we have been looking at the wrong part of the window,” Wong said. “While most clinical eye exams focus on the central retina, the most critical early indicators of AD appear to be hidden at the periphery of the eye. By identifying these retinal changes that occur before the brain’s ‘plumbing’ system fails, doctors may eventually be able to use routine eye exams to catch and treat the disease years before memory loss begins.”


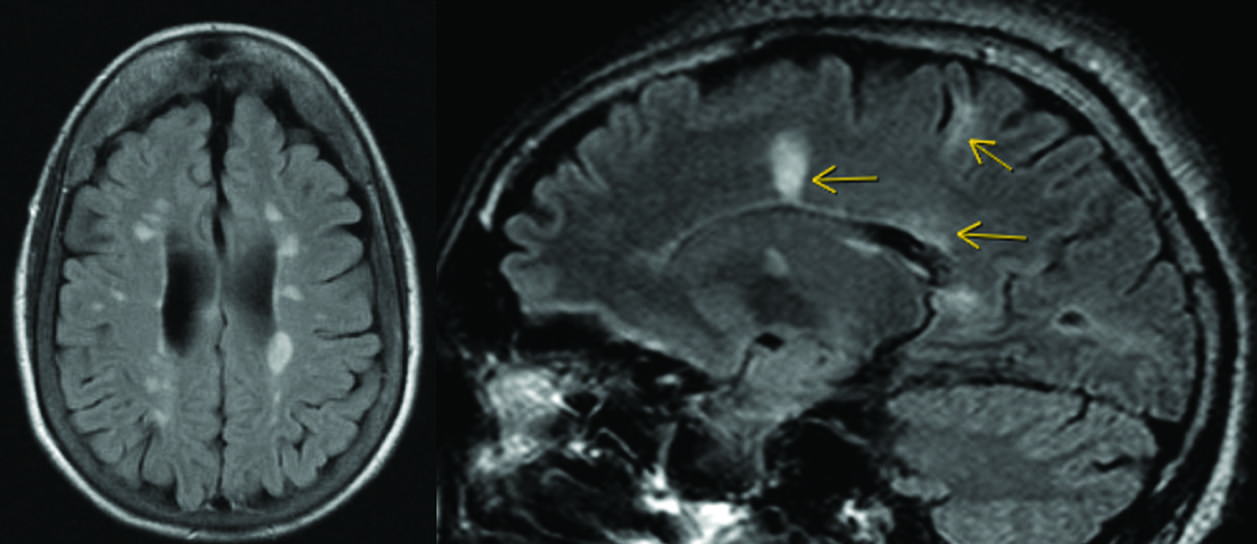
MRI is central to modern MultipleSclerosis diagnosis.
A review article by Drs. Elfasi and Fagundo highlights radiologic lbiomarkers in the 2024 McDonald criteria—including the central vein sign, paramagnetic rim lesions, cortical lesions, and optic nerve imaging.
https://ow.ly/cZrC50Yj69O National Multiple Sclerosis Society ACTRIMS American Academy of Neurology (AAN) Radiological Society of North America (RSNA) University of South Florida.
Imaging biomarkers, including cortical lesions, the central vein sign, and paramagnetic rim lesions, allow for more timely and accurate diagnosis of multiple sclerosis.

Immune suppression largely contributes to cancer occurrence and progression. The programmed cell death protein 1 (PD-1, also known as PDCD1 and CD279) was originally identified by Ishida et al. in apoptotic mouse T-cell tumors [1]. PD-1 is a transmembrane protein belonging to the CD28/CTLA-4 superfamily. It is widely expressed at the surface of activated T cells, B cells, monocytes, and other immune cells, and negatively regulates human immune response through binding with its two ligands, namely programmed cell death 1 ligands (PD-L1 or PD-L2). PD-L1 (B7-H1; CD274) and PD-L2 (B7-DC; CD273) belong to the B7 family of T cell co-inhibitory molecules. PD-L1 is widely expressed in antigen-presenting cells and tissues, such as heart and lung [2, 3]. The interaction of PD-1 with PD-L1 or PD-L2 provides inhibitory signals responsible for inhibiting T cell signaling, mediating the mechanisms of tolerance, and providing immune homeostasis. Therefore, PD-1 suppresses autoimmunity and prevents the occurrence of autoimmune diseases. In addition, PD-L1 or PD-L2 expressed by cancer cells binds to PD-1 on the surface of T cells, thereby inhibiting T cell activation and leading to cancer immune escape [4]. Numerous studies revealed that PD-L1 expression is very high in lung cancer, melanoma, glioma, breast cancer and other malignant tumor cells, forming an immunosuppressive tumor microenvironment [5].
PD-1 mainly consists of extracellular IgV-like domain region, hydrophobic transmembrane region and cytoplasmic region, and the tail of the cytoplasmic region has immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) [6, 7], which is an important structural basis for PD-1 to transmit inhibitory signals and perform immunosuppressive functions. PD-L1 is structurally similar to PD-1 and is more conserved and widely expressed than PD-L2 [8], so it plays the leading effect in tumor cells immune evasion. In recent years, antagonistic antibodies against PD-1 or PD-L1 have been approved by the FDA to treat cancer, opening a new chapter in tumor immunotherapy across the era [9].
Anti-PD-1/PD-L1 inhibitors have become effective immune checkpoint inhibitors (ICIs) and are rapidly becoming the standard therapy for various cancers. Tumor immunotherapy aims to block the activity of inhibitory immune checkpoint proteins and promote T cell activation to achieve anti-tumor immune effects [10]. Owing to their safety and precision, these inhibitors hold significant promise in tumor immunotherapy. Research indicates that the PD-1/PD-L1 pathway plays a crucial role in regulating autoimmunity responses and peripheral tolerance. Notably, anti-PD-1/PD-L1 immunotherapy can effectively block the PD-1/PD-L1 signaling pathway, restore T cell activity, enhance anti-tumor immunity, and then eliminate tumor cells [11, 12]. Therefore, the discovery of multiple immunotherapies, such as PD-1 and PD-L1 inhibitors, has significant clinical implications for tumor-specific immunotherapy.

Experimental models such as heterochronic parabiosis and heterochronic plasma transfer have profoundly advanced our understanding of systemic aging, demonstrating that circulating factors can influence brain, vascular, and immune aging through cell nonautonomous mechanisms. These preclinical models have revealed that both pro-geronic and anti-geronic signals in blood can modulate neuroinflammation, neurovascular health, and cognitive resilience. However, despite their experimental promise, the clinical translation of these findings, particularly through plasma-based interventions in humans, remains fraught with uncertainty.
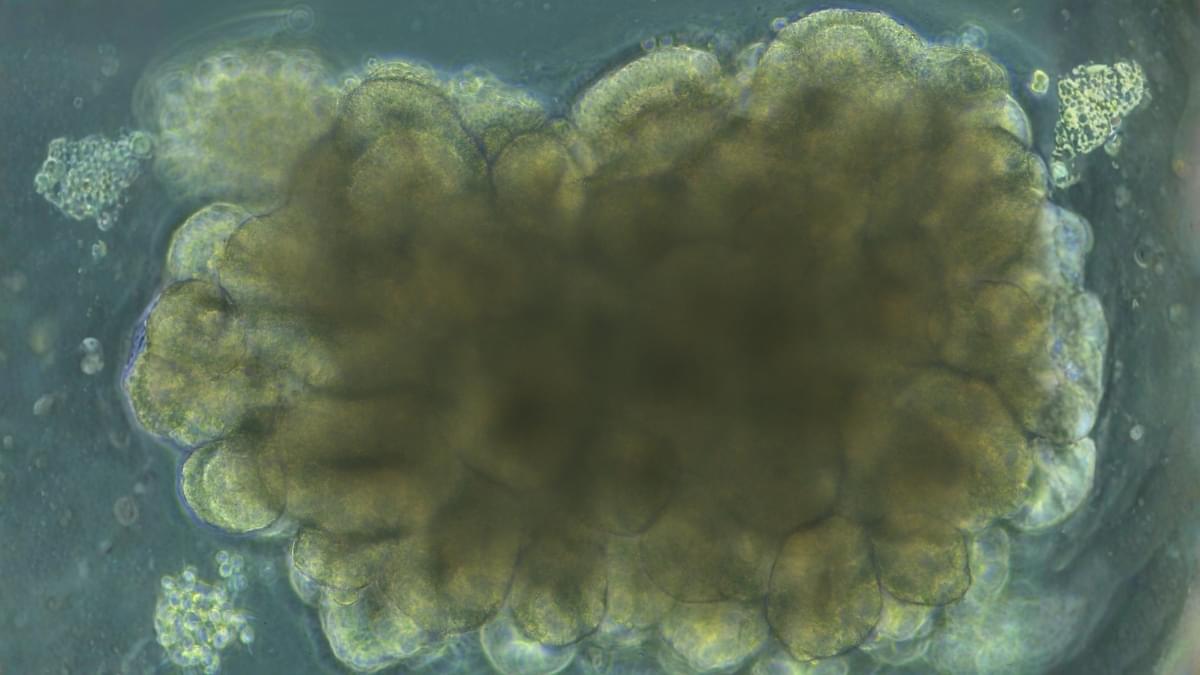
A few blobs of lab-grown brain tissue have demonstrated a striking proof of concept: living neural circuits can be nudged toward solving a classic control problem through carefully structured feedback.
In a closed-loop system that delivered electrical feedback based on performance, cortical organoids could steadily improve their control of a classic engineering benchmark: balancing an unstable virtual pole.
The improvement is far from a functioning hybrid biocomputer. But as a proof of concept, it shows that neural tissue in a dish can be adaptively tuned through structured feedback – a result that could help researchers probe how neurological disease alters the brain’s capacity for plasticity.
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
Blood testing with LifeExtension.com: https://www.anrdoezrs.net/click-101614996-15750394
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1