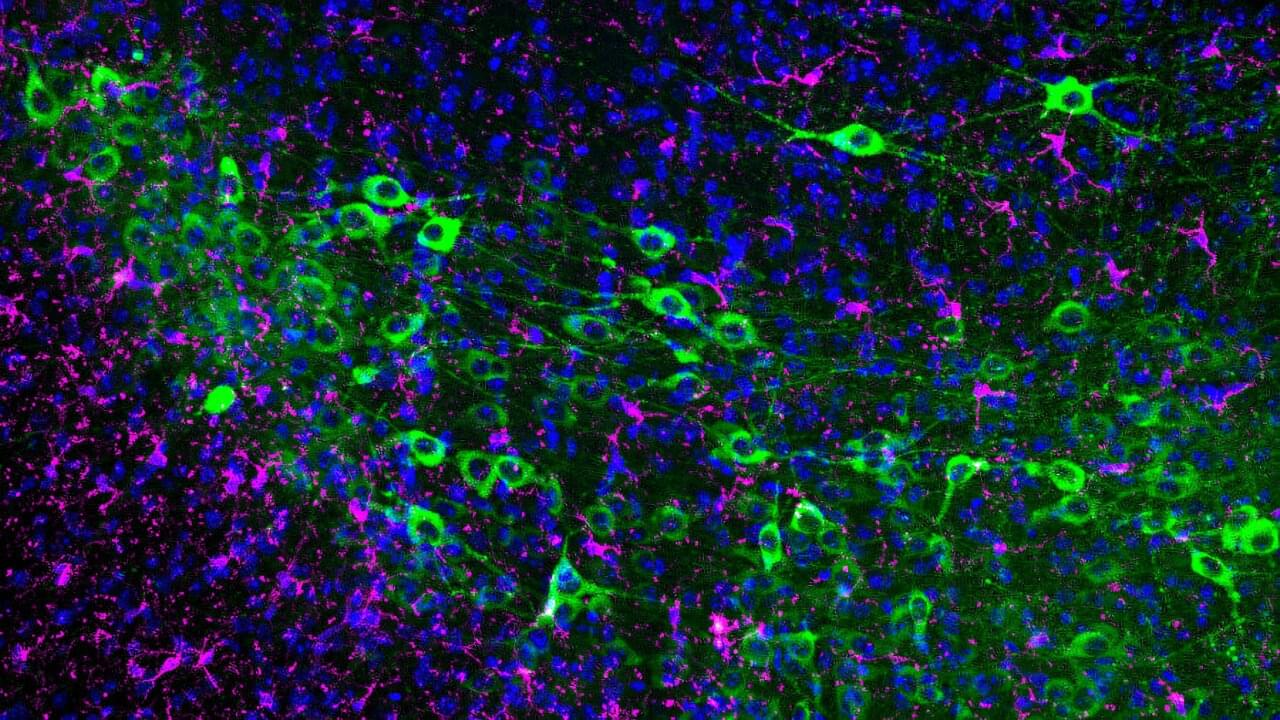
This screening platform washed cells with a broad range of retroviruses to determine which ones affect tau. In follow-up testing, the gene CUL5 was singled out as being crucial for tau degradation. Mitochondrial function was also found to be a key part of preventing tau pathology.
Using an ingenious CRISPR-based screening technique, scientists have found a protein that tags tau for degradation and is more strongly expressed in tau-resilient neurons [1].
Some neurons are more equal than others
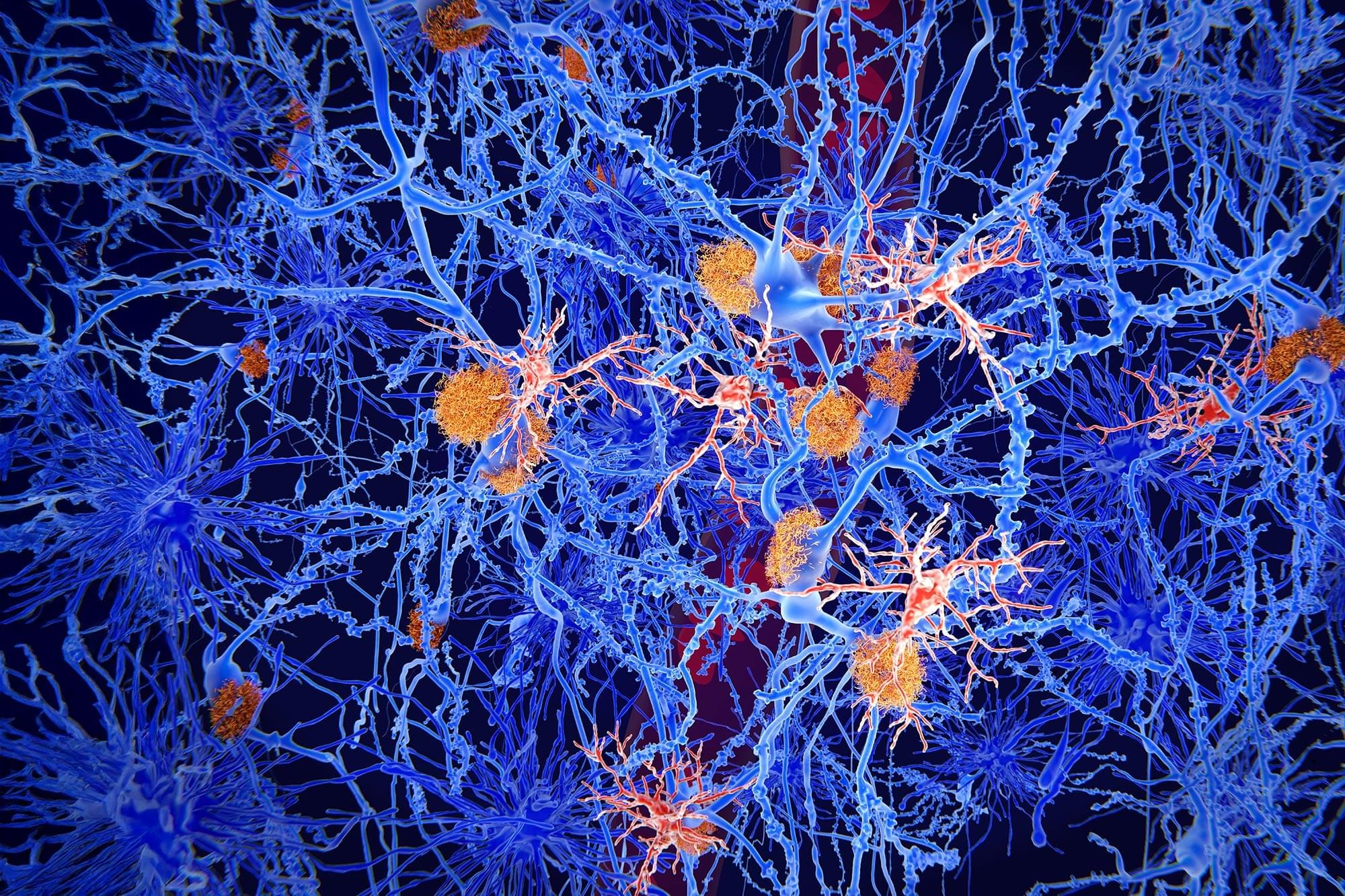
The accumulation of tau protein fibrils in neurons is a hallmark of Alzheimer’s and several other diseases [2]. Scientists have long noticed that even in the brains of people who died of Alzheimer’s, some neurons are markedly healthier than others, suggesting that neurons differ in how they handle tau and that these differences may explain selective vulnerability in tauopathies [3].