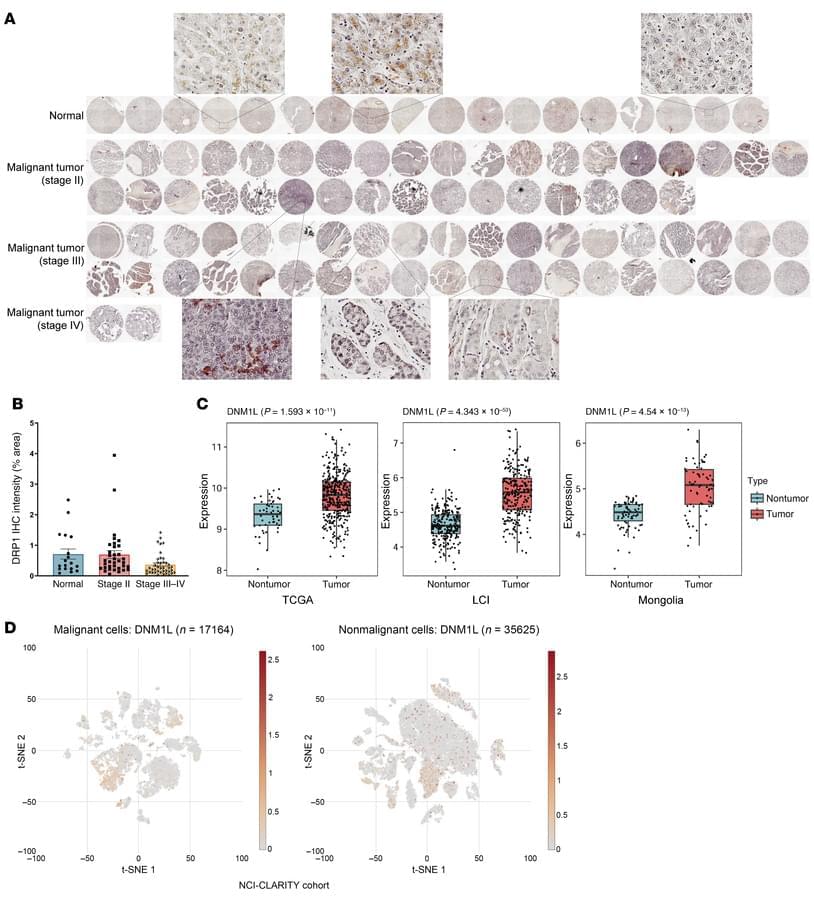
Objective: To enhance vascular-targeted photodynamic therapy (V-PDT) efficacy by integrating real-time dosimetric monitoring and adaptive irradiance modulation based on dynamic physiological feedback. Impact Statement: This study presents a closed-loop, dual-modality optical imaging-guided V-PDT platform that enables individualized, oxygen-informed irradiance control, improving therapeutic precision and efficiency. Introduction: While V-PDT is a promising, minimally invasive treatment for tumors and vascular abnormalities, its efficacy is often hindered by rapid oxygen depletion under high irradiance, leading to treatment-limiting hypoxia. Accurate, real-time assessment of both photosensitizer concentration and blood oxygenation is essential to guide optimized therapeutic strategies, yet such capability has remained elusive in clinical settings.