npj Biological Physics and Mechanics volume 2, Article number: 3 (2025) Cite this article.

npj Biological Physics and Mechanics volume 2, Article number: 3 (2025) Cite this article.
The musk blueprint: navigating the supersonic tsunami to hyperabundance when exponential curves multiply: understanding the triple acceleration.
On January 22, 2026, Elon Musk sat down with BlackRock CEO Larry Fink at the World Economic Forum in Davos and delivered what may be the most important articulation of humanity’s near-term trajectory since the invention of the internet.
Not because Musk said anything fundamentally new—his companies have been demonstrating this reality for years—but because he connected the dots in a way that makes the path to hyperabundance undeniable.
[Watch Elon Musk’s full WEF interview]
This is not visionary speculation.
This is engineering analysis from someone building the physical infrastructure of abundance in real-time.
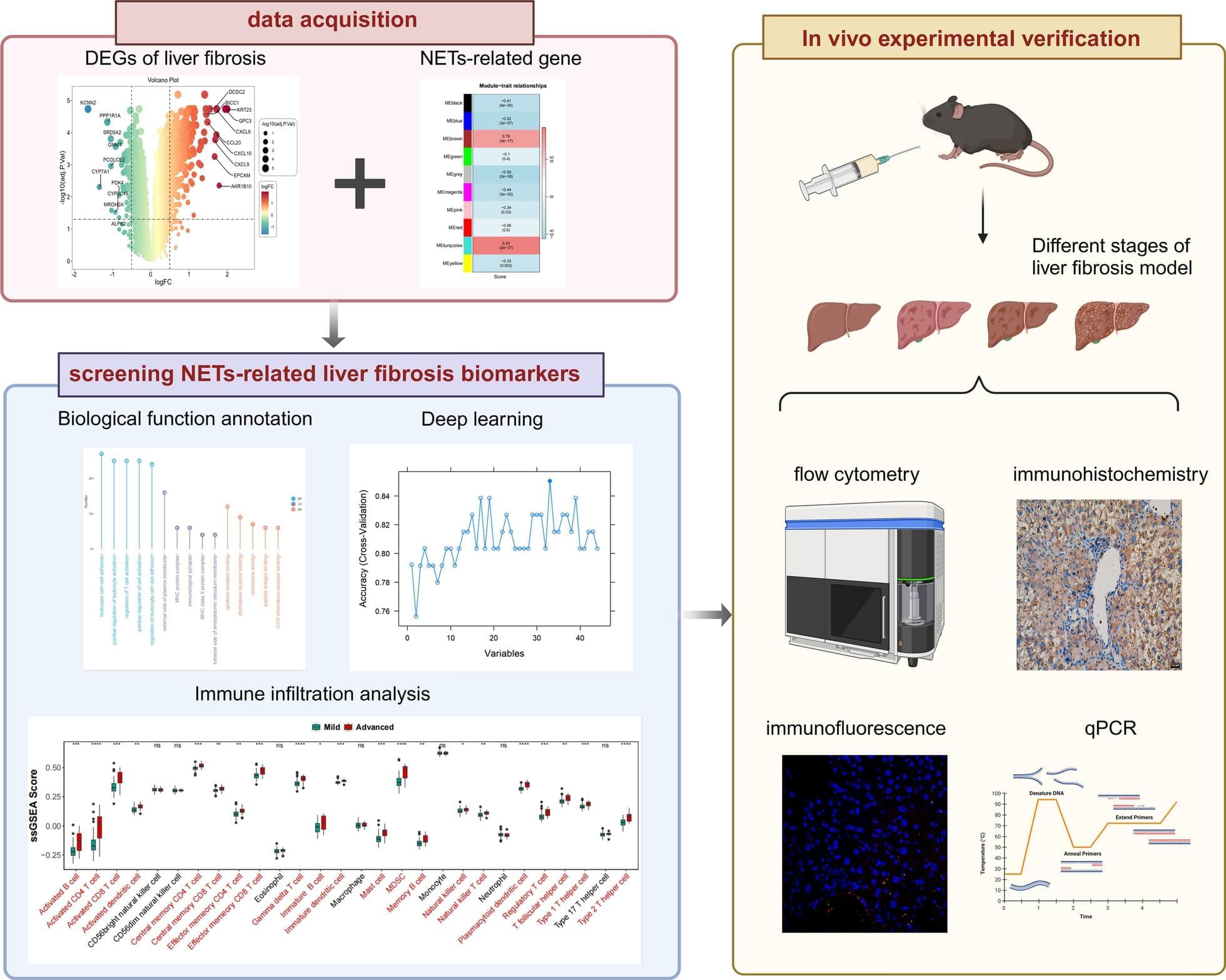
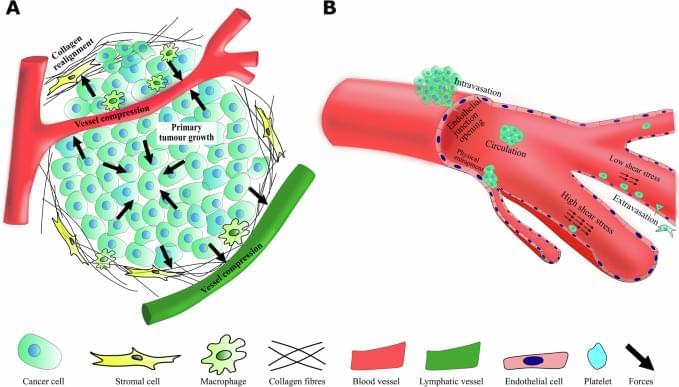
The diagnostic and therapeutic potential of neutrophil extracellular traps (NETs) in liver fibrosis (LF) has not been fully explored. We aim to screen and verify NETs-related liver fibrosis biomarkers through machine learning.
In order to obtain NETs-related differentially expressed genes (NETs-DEGs), differential analysis and WGCNA analysis were performed on the GEO dataset (GSE84044, GSE49541) and the NETs dataset. Enrichment analysis and protein interaction analysis were used to reveal the candidate genes and potential mechanisms of NETs-related liver fibrosis. Biomarkers were screened using SVM-RFE and Boruta machine learning algorithms, followed by immune infiltration analysis. A multi-stage model of fibrosis in mice was constructed, and neutrophil infiltration, NETs accumulation and NETs-related biomarkers were characterized by immunohistochemistry, immunofluorescence, flow cytometry and qPCR. Finally, the molecular regulatory network and potential drugs of biomarkers were predicted.
A total of 166 NETs-DEGs were identified. Through enrichment analysis, these genes were mainly enriched in chemokine signaling pathway and cytokine-cytokine receptor interaction pathway. Machine learning screened CCL2 as a NETs-related liver fibrosis biomarker, involved in ribosome-related processes, cell cycle regulation and allograft rejection pathways. Immune infiltration analysis showed that there were significant differences in 22 immune cell subtypes between fibrotic samples and healthy samples, including neutrophils mainly related to NETs production. The results of in vivo experiments showed that neutrophil infiltration, NETs accumulation and CCL2 level were up-regulated during fibrosis. A total of 5 miRNAs, 2 lncRNAs, 20 function-related genes and 6 potential drugs were identified based on CCL2.

A former professor at the University of California, Los Angeles, Horvath is now principal investigator of the U.K. research arm of Altos Labs, a longevity biotech company that says it is developing therapies that could reverse age-related diseases and disabilities.
Having precise and meaningful ways to measure aging could make it possible for drug developers like Altos Labs to test longevity treatments in people, Horvath says: “It’s a quintessential tool to find interventions for rejuvenation.”
As part of TIME’s series interviewing longevity leaders and influencers, we spoke to Horvath about his pioneering invention and what he thinks might be possible for human life extension.
As we age, the immune system gradually declines in function, leaving the body more vulnerable to disease. Scientists have discovered a new way to rejuvenate a key component of immune function, potentially boosting health in later years.
A team from the Broad Institute of MIT and Harvard focused on the thymus, a small organ in front of the heart that’s crucial for the development of T cells. These immune cells act as guards, identifying and fighting threats such as cancer and infections.
From early adulthood, the thymus shrinks and slows, limiting T cell production. In mouse models, the researchers were able to repurpose part of the liver as a thymus substitute, sending the molecular signals that stimulate T-cell production.
Lung cancer varies widely from patient to patient, and that diversity makes it hard to find effective treatments. Researchers at the Berlin Institute of Health at Charité (BIH) have developed a method to evaluate multiple therapeutic approaches on patient-derived “tumoroids”—miniature tumors grown from tissue removed during surgery at Charité
By testing drug responses across these tumoroids, the team showed that therapeutic success depends on a complex interplay of tumor characteristics rather than a single factor. Their results suggest that tumoroid-based testing could help physicians tailor treatments to individual patients and improve clinical decision-making.
The BIH researchers have published their findings in Nature Biomedical Engineering.
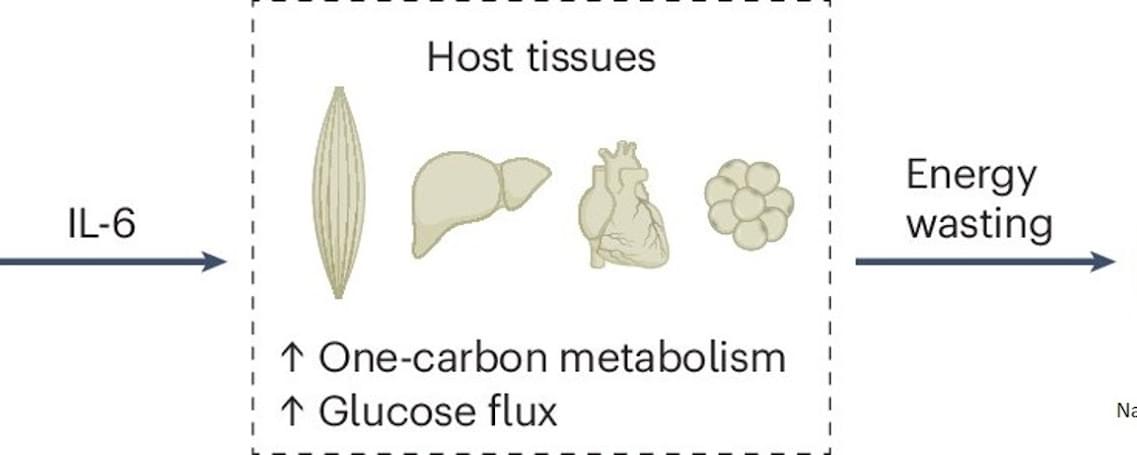
Combined metabolomics and transcriptomics analysis in eight different organs of tumor-bearing mice with and without cachexia allowed researchers to create metabolic signatures typical of cancer-associated weight loss. High-throughput analyses identified a cachexia-specific metabolic and genetic signature that provides insight into the progression of these metabolic changes.
The researchers found that all organs showed increased activation of the so-called “one carbon cycle”, a biochemical process essential for the synthesis of nucleotides, amino acids, and cell regeneration. Products of this cycle, such as sarcosine or dimethylglycine, could potentially serve as biomarkers for cachexia in the future.
The study also revealed that hyperactivation of the one carbon cycle in muscle is associated with increased glucose metabolism (glucose hypermetabolism) and muscle atrophy. Early experiments suggest that inhibiting this process could prevent muscle loss. Comparative analyses across eight different mouse tumor models (lung, colon, and pancreatic cancer) confirmed that the one carbon signature represents a universal cachexia signature, independent of cancer type.
Currently, there is no approved drug for cancer cachexia in Germany. New approaches are being explored to address cancer-related appetite loss. This study provides the first evidence of how metabolism itself could potentially be normalized. Early experiments in cell cultures show that interventions targeting the one carbon cycle can have positive effects. sciencenewshighlights ScienceMission.
Cachexia is a metabolic disorder that causes uncontrolled weight loss and muscle wasting in chronic diseases and cancer. A new study shows that cachexia affects more than just muscles. Numerous organs respond in a coordinated manner, ultimately contributing to muscle loss. Analysis of metabolome and transcriptome data, along with glucose tracing in tumor-bearing mouse models, identified a novel mechanism that plays a key role in cancer-associated weight loss.
A loss of 10% of body weight within six months – what may sound desirable in some contexts – often causes uncertainty and frustration in cancer patients with cachexia, as they are unable to maintain or gain body weight despite wanting to. Cachexia (from the Greek kakós, “bad,” and héxis, “condition”) affects 50–80% of all cancer patients, reduces quality of life, diminishes the effectiveness of cancer therapies, and increases mortality.

Throwing another log into a crackling fireplace on a cold winter’s night might seem like a cozy, harmless tradition. But Northwestern University scientists have found residential wood burning is a major—yet often overlooked—contributor to winter air pollution across the United States.
Although only 2% of U.S. homes rely on wood as their primary heating source, residential wood burning accounts for more than one-fifth of Americans’ wintertime exposure to outdoor fine particulate matter (PM2.5), the new study found.
These tiny airborne particles can penetrate deep into the lungs and enter the bloodstream, where they are linked to increased risks of heart disease, lung disease and even premature death. Among their findings, the scientists calculated that pollution from residential wood burning is associated with about 8,600 premature deaths per year.