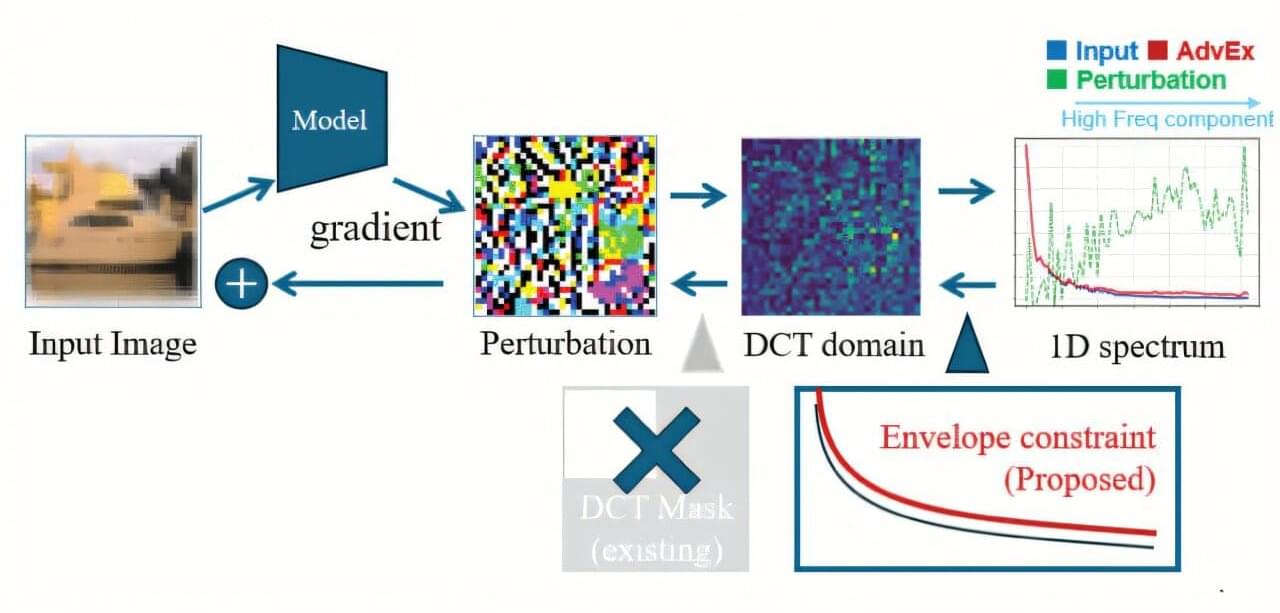
Researchers at Mount Sinai have developed a novel immunotherapy strategy that targets the tumor microenvironment (TME) to overcome immune suppression in metastatic cancers. Addressing the protective role of tumor-associated macrophages (TAMs), which often shield malignancies and facilitate growth, the team engineered chimeric antigen receptor (CAR) T-cells to specifically recognize and target these stromal cells. Functioning as a “Trojan horse,” these modified T-cells not only engage macrophages but also release immune-activating molecules that reprogram the TME, converting immunosuppressive macrophages into anti-tumor effectors. In preclinical models of metastatic lung and ovarian cancer, this approach yielded significant therapeutic efficacy, resulting in extended survival and the complete eradication of tumors in some subjects. By transforming the tumor’s protective infrastructure into a mechanism of its destruction, this strategy offers a promising, potentially pan-cancer modality for treating solid tumors resistant to conventional immunotherapies.
Scientists at the Icahn School of Medicine at Mount Sinai have developed an experimental immunotherapy that takes an unconventional approach to metastatic cancer: instead of going after cancer cells directly, it targets the cells that protect them.
T he study, published in the January 22 online issue of Cancer Cell, a Cell Press Journal [DOI 10.1016/j.ccell.2025.12.021], was conducted in aggressive preclinical models of metastatic ovarian and lung cancer. It points to a new strategy for treating advanced-stage solid tumors.
In a strategy modeled after the famed Trojan horse, the treatment enters the tumors by targeting cells called macrophages that guard the cancer cells, disarms these protectors, and opens up the tumor’s gates for the immune system to enter and wipe out the cancer cells.