Researchers will be able to analyze chemical compounds and atoms in greater detail than ever before using the brightest, clearest laser of its kind anywhere in the world.


All proteins are composed of chains of amino acids, which generally fold up into compact globules with specific shapes. The folding process is governed by interactions between the different amino acids—for example, some of them carry electrical charges—so the sequence determines the structure. Because the structure in turn defines a protein’s function, deducing a protein’s structure is vital for understanding many processes in molecular biology, as well as for identifying drug molecules that might bind to and alter a protein’s activity.
Protein structures have traditionally been determined by experimental methods such as x-ray crystallography and electron microscopy. But researchers have long wished to be able to predict a structure purely from its sequence—in other words, to understand and predict the process of protein folding.
For many years, computational methods such as molecular dynamics simulations struggled with the complexity of that problem. But AlphaFold bypassed the need to simulate the folding process. Instead, the algorithm could be trained to recognize correlations between sequence and structure in known protein structures and then to generalize those relationships to predict unknown structures.
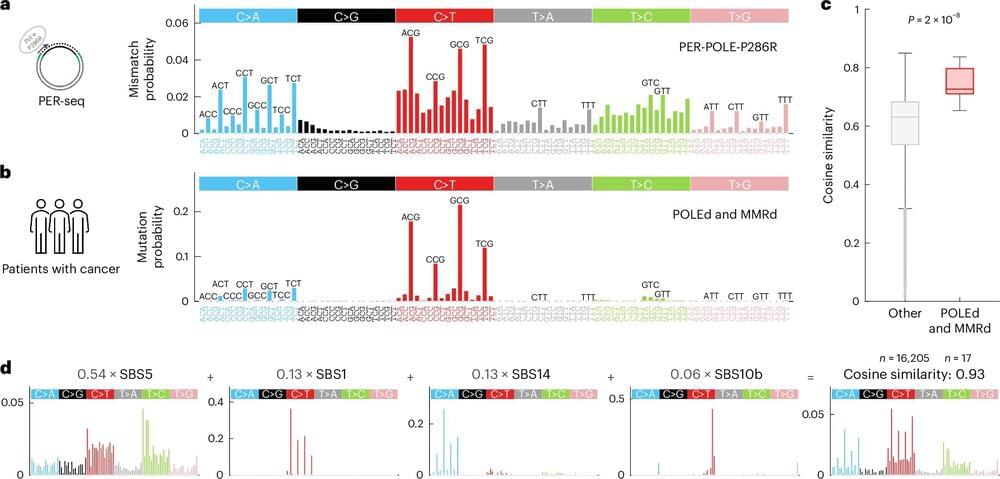
A Ludwig Cancer Research study has punctured a longstanding assumption about the source of the most common type of DNA mutation seen in the genome—one that contributes to many genetic diseases, including cancer.
Led by Ludwig Oxford Leadership Fellow Marketa Tomkova, postdoc Michael McClellan, Assistant Member Benjamin Schuster-Böckler and Associate Investigator Skirmantas Kriaucionis, the study has implications not only for basic cancer biology but also for such things as assessments of carcinogenic risk associated with environmental factors and our understanding of the emergence of drug resistance during cancer therapy. Its findings are reported in the current issue of Nature Genetics.
The mutation in question—in which cytosine ©, one of the four bases of DNA that spell out our genes, is erroneously switched to thymine (T)—was thought to be primarily the result of a spontaneous chemical reaction with water. This reaction, deamination, is about twice as likely to happen when a cytosine is chemically tagged by the addition of a molecule known as a methyl group to create 5-methylcytosine, which occurs in DNA at so-called “CpG” positions, where C is followed by the base guanine (G).

Researchers have developed a Martian atmospheric evolution model to propose a new theory about Mars’s past. Although Mars is currently a cold, dry planet, geological evidence suggests that liquid water existed there around 3 to 4 billion years ago. Where there is water, there is usually life. In their quest to answer the burning question about life on Mars, researchers at Tohoku University created a detailed model of organic matter production in the ancient Martian atmosphere.
Organic matter refers to the remains of living things such as plants and animals, or the byproduct of certain chemical reactions.
Whatever the case, the stable carbon isotope ratio (13C/12C) found in organic matter provides valuable clues about how these building blocks of life were originally formed, giving scientists a window into the past.
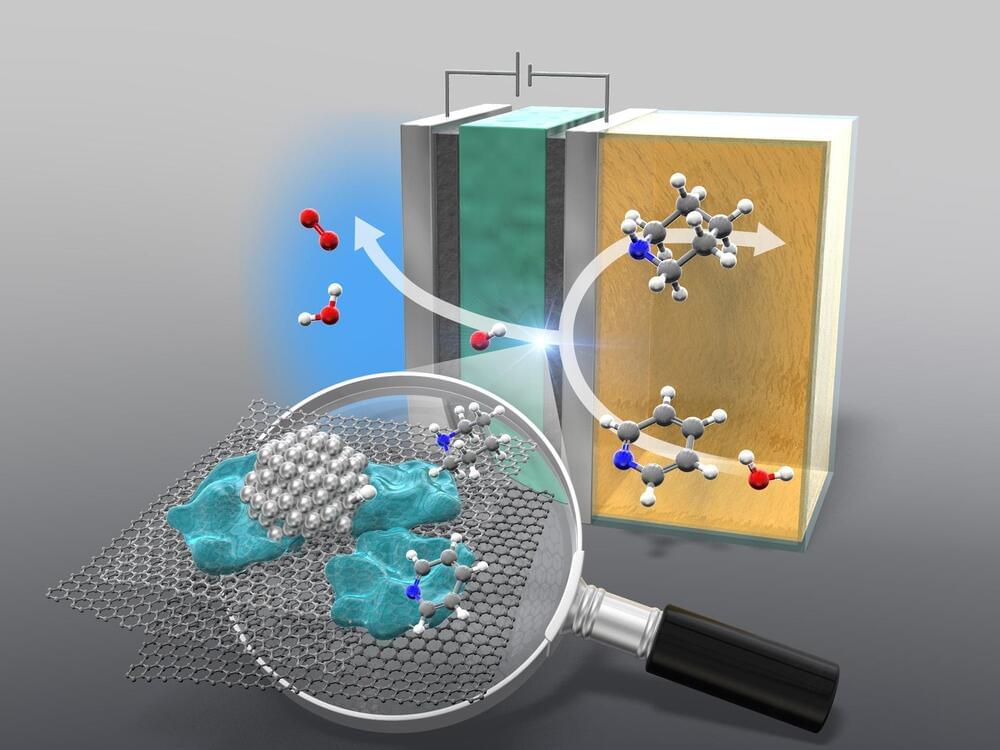
A new study introduces an eco-friendly method using an AEM electrolyzer to hydrogenate cyclic amines, reducing the chemical industry’s carbon emissions. This process replaces fossil fuels with water and renewable electricity, maintaining high efficiency.
To reduce the environmental impact of the chemical manufacturing industry, it is crucial to develop greener methods for producing the chemical building blocks of widely used compounds.
It’s no secret manufacturing processes have some of the most impactful and intense effects on the environment, with the chemical manufacturing industry topping the charts for both energy consumption and emissions output. While this makes sense thanks to the grand scale in which manufactured chemicals are involved in daily life, it still leaves a lot to be desired for sustainability’s sake. By focusing on renewable energy sources and alternative methods for creating the chemical building blocks of some of the most commonly used compounds, researchers hope to reduce the chemical manufacturing industry’s footprint with some green innovation.

The solutions to these long-standing problems could further enhance our understanding of symmetries of structures and objects in nature and science, and of long-term behavior of various random processes arising in fields ranging from chemistry and physics to engineering, computer science and economics.
A Rutgers University-New Brunswick professor who has devoted his career to resolving the mysteries of higher mathematics has solved two separate, fundamental problems that have perplexed mathematicians for decades.
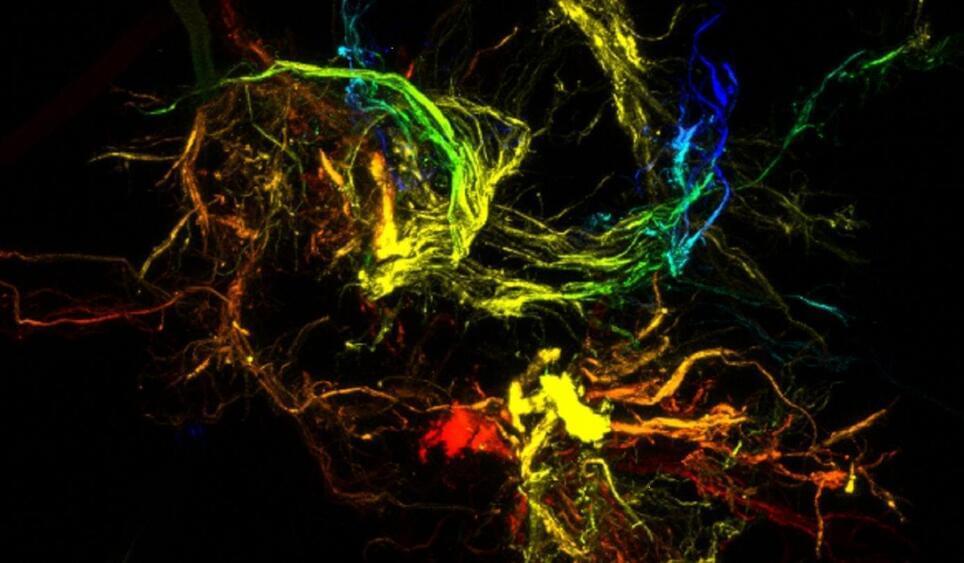
New research from North Carolina State University shows that unique materials with distinct properties akin to those of gecko feet – the ability to stick to just about any surface – can be created by harnessing liquid-driven chaos to produce soft polymer microparticles with hierarchical branching on the micro-and nanoscale.
The findings, published today (October 14, 2019) in the journal Nature Materials, hold the potential for advances in gels, pastes, foods, nonwovens, and coatings, among other formulations.
The soft dendritic particle materials with unique adhesive and structure-building properties can be created from a variety of polymers precipitated from solutions under special conditions, says Orlin Velev, S. Frank and Doris Culberson Distinguished Professor of Chemical and Biomolecular Engineering at NC State and corresponding author of the paper.