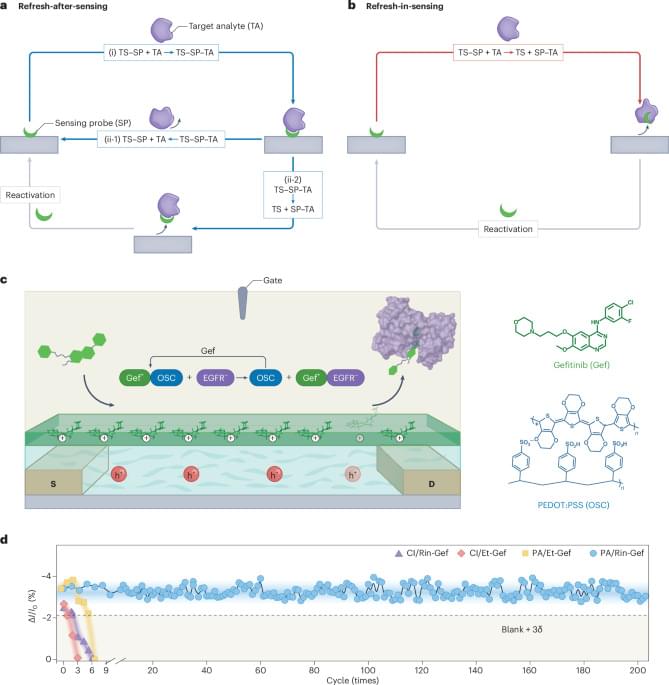
An electrochemical biosensor capable of detecting low levels of cancer biomarkers is reusable over 200 regeneration cycles without compromising device sensitivity and accuracy.

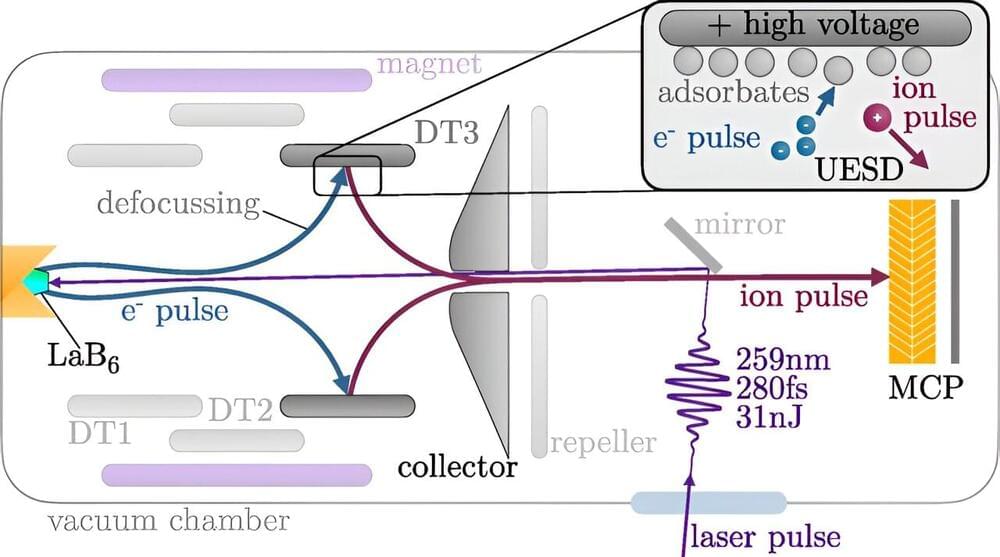


However, more recent research suggests there are likely countless other possibilities for how life might emerge through potential chemical combinations. As the British chemist Lee Cronin, the American theoretical physicist Sara Walker and others have recently argued, seeking near-miraculous coincidences of chemistry can narrow our ability to find other processes meaningful to life. In fact, most chemical reactions, whether they take place on Earth or elsewhere in the Universe, are not connected to life. Chemistry alone is not enough to identify whether something is alive, which is why researchers seeking the origin of life must use other methods to make accurate judgments.
Today, ‘adaptive function’ is the primary criterion for identifying the right kinds of biotic chemistry that give rise to life, as the theoretical biologist Michael Lachmann (our colleague at the Santa Fe Institute) likes to point out. In the sciences, adaptive function refers to an organism’s capacity to biologically change, evolve or, put another way, solve problems. ‘Problem-solving’ may seem more closely related to the domains of society, culture and technology than to the domain of biology. We might think of the problem of migrating to new islands, which was solved when humans learned to navigate ocean currents, or the problem of plotting trajectories, which our species solved by learning to calculate angles, or even the problem of shelter, which we solved by building homes. But genetic evolution also involves problem-solving. Insect wings solve the ‘problem’ of flight. Optical lenses that focus light solve the ‘problem’ of vision. And the kidneys solve the ‘problem’ of filtering blood. This kind of biological problem-solving – an outcome of natural selection and genetic drift – is conventionally called ‘adaptation’. Though it is crucial to the evolution of life, new research suggests it may also be crucial to the origins of life.
This problem-solving perspective is radically altering our knowledge of the Universe. Life is starting to look a lot less like an outcome of chemistry and physics, and more like a computational process.

McGill University researchers have harnessed the power of sunlight to transform two of the most harmful greenhouse gases into valuable chemicals. The discovery could help combat climate change and provide a more sustainable way to produce certain industrial products.
“Imagine a world where the exhaust from your car or emissions from a factory could be transformed, with the help of sunlight, into clean fuel for vehicles, the building blocks for everyday plastics, and energy stored in batteries,” said co-first author Hui Su, a Postdoctoral Fellow in McGill’s Department of Chemistry. “That’s precisely the kind of transformation this new chemical process enables.”
The research team’s new light-driven chemical process converts methane and carbon dioxide into green methanol and carbon monoxide in one reaction. Both products are highly valued in the chemical and energy sectors, the researchers said.
A flexible screen inspired in part by squid can store and display encrypted images like a computer—using magnetic fields rather than electronics. The research is reported in Advanced Materials by University of Michigan engineers.
“It’s one of the first times where mechanical materials use magnetic fields for system-level encryption, information processing and computing. And unlike some earlier mechanical computers, this device can wrap around your wrist,” said Joerg Lahann, the Wolfgang Pauli Collegiate Professor of Chemical Engineering and co-corresponding author of the study.
The researchers’ screen could be used wherever light and power sources are cumbersome or undesirable, including clothing, stickers, ID badges, barcodes and e-book readers. A single screen can reveal an image for everyone to see when placed near a standard magnet or a private encrypted image when placed over a complex array of magnets that acts like an encryption key.
The Road To Wisdom — Dr. Francis Collins, MD, PhD — Former Director, National Institutes of Health (NIH); Distinguished Investigator, Center for Precision Health Research, National Human Genome Research Institute.
Dr. Francis S. Collins, M.D., Ph.D., (https://www.francisscollins.com/) is the former Director of the U.S. National Institutes of Health (NIH), where as the longest serving director of NIH (spanning 12 years and three presidencies) he oversaw the work of the largest supporter of biomedical research in the world, from basic to clinical research.
Dr. Collins continues to serve as NIH Distinguished Investigator.
Center for Precision Health Research, at the National Human Genome Research Institute (NHGRI — https://irp.nih.gov/pi/francis-collins).
Dr. Collins is a physician-geneticist noted for his landmark discoveries of disease genes and his leadership of the international Human Genome Project, which culminated in April 2003 with the completion of a finished sequence of the human DNA instruction book. He served as director of the National Human Genome Research Institute at the NIH from 1993–2008.
Dr. Collins’ research laboratory has discovered a number of important genes, including those responsible for cystic fibrosis, neurofibromatosis, Huntington’s disease, a familial endocrine cancer syndrome, and most recently, genes for type 2 diabetes, and the gene that causes Hutchinson-Gilford progeria syndrome, a rare condition that causes premature aging.

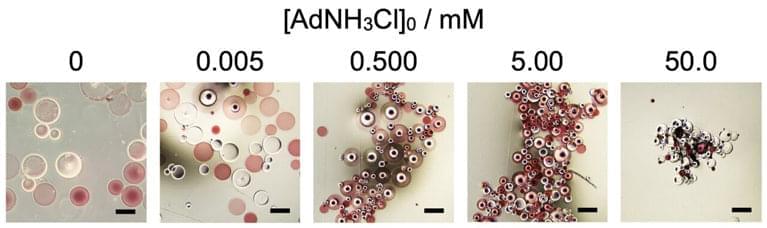
If you’ve ever opened a box from IKEA and wished the pieces inside could somehow spontaneously merge to form a table or chair, then a simple virus could have a thing or two to teach you. Self-assembly of complex molecules is essential for a wide array of biological structures, including proteins, cell membranes, or even entire viruses. Supramolecular chemistry is a field of study that attempts to build large molecules out of a discrete number of…
NEW PAPER — Loss of the Primal Eye in evolution, REM explained as phasic transients, and the emergence of DREAMING in E1 animals. MA dissertation Philosophy, University of Leeds 1995/1996.
There are a number of reasons why dreaming has been, and remains, an important area to philosophy. Dreams are ‘pure’ experiential phenomena not (seemingly) requiring input from the outside world via the special senses. As Aristotle puts it, “If all creatures, when the eyes are closed in sleep, are unable to see, and the analogous statement is true of the other senses, so that manifestly we perceive nothing when asleep; we may conclude that it is not by sense-perception we perceive a dream”. A major part of this dissertation is concerned with issues raised in Owen Flanagan’s (1995) article, Deconstructing Dreams: The Spandrels of Sleep. The Primal Eye/MVT account of consciousness gives p-dreaming a more central explanatory role, and I argue that p-dreams are not epiphenomena in the way Flanagan claims. An important omission from Flanagan’s account is any discussion of important dreaming-related phenomena. I look at lucid dreaming, hypnosis and other mental phenomena in relation to the evolutionary loss of the primal/ median/ parietal eye, and postulate that REM rapid eye movements are ‘phasic transients’ considering the E1 brain which includes the lateral eyes, as a consciousness-producing circuit. A brief account of Primal Eye/ Median Vision Theory is that capacity for abstract/ centrally evoked mentation is a direct result of the evolutionary loss of the primal eye. E2 (earlier hardwired brains with both primal and lateral eyes) have evolved over millions of years into E1 brain circuits analog(ous to infinite-state) types of self-regulating plastic circuits, with no primal/pineal eye, but retaining lateral eyes and the pineal gland. Loss of this ‘lockstep mechanism’ median/primal/ parietal/pineal eye not only allowed new sleeping mental phenomena such as dreaming; but also heralded in new types of waking mental abstraction freed from E2 involuntary primal eye direct (electro-chemical) responses to changes in the physical environment. These include daydreams, visualisation with both lateral eyes closed, self-volition or self-determined choices, and so on.
See Full PDF