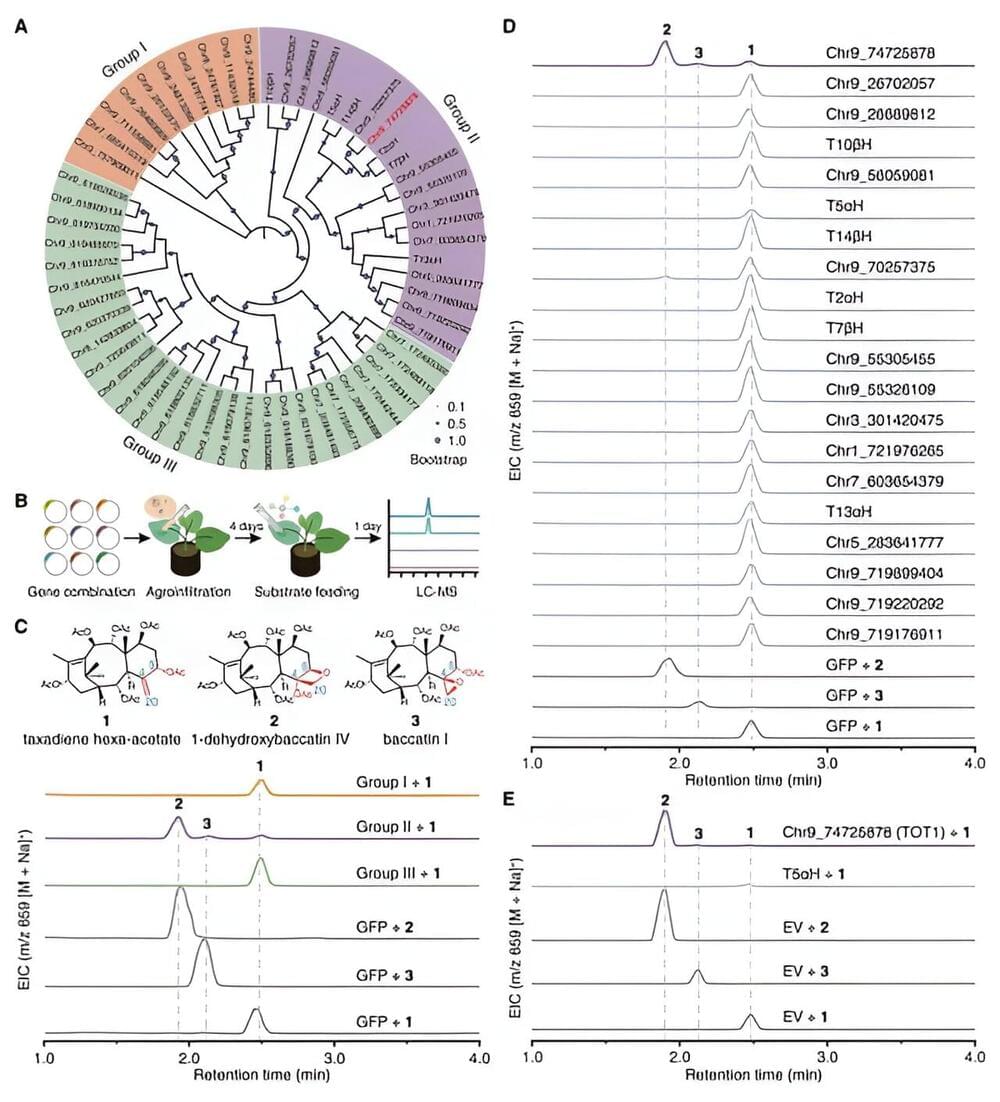
Paclitaxel is the world’s best-selling plant-based anticancer drug and one of the most effective anticancer drugs over the past 30 years. It is widely used in the treatment of various types of cancer, including breast cancer, lung cancer, and ovarian cancer.
In the late 1990s and early 21st century, the annual sales of paclitaxel exceeded $1.5 billion and reached $2.0 billion in 2001, making it the best-selling drug in 2001. In 2019, the market for paclitaxel and its derivatives was approximately $15 billion, and it is expected to reach $20 billion by 2025.
As an anticancer drug, the molecular structure of paclitaxel is extremely complex, with highly oxidized, intricate bridged rings and 11 stereocenters, making it widely recognized as one of the most challenging natural products to synthesize chemically. Since the first total synthesis of paclitaxel was reported by the Holton and Nicolaou research groups in 1994, more than 40 research teams have been engaged in the total synthesis of paclitaxel.