Blood–brain barrier: A physical and biochemical boundary between the bloodstream and the parenchyma of the central nervous system (CNS).
Editorial from The New England Journal of Medicine — Sounding Out the Blood–Brain Barrier.
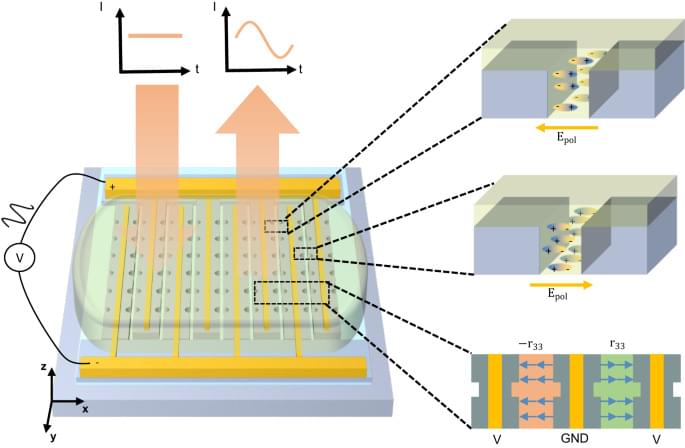
Relying on sub-wavelength nanostructures, metasurfaces have been shown as promising candidates for replacing conventional free-space optical components by arbitrarily manipulating the amplitude, phase, and polarization of optical wavefronts in certain applications1,2,3. In recent years, the scope of their applications has been expanded towards complete spatio-temporal control through the introduction of active metasurfaces. These developments open up exciting new possibilities for dynamic holography4, faster spatial light modulators5, and fast optical beam steering for LiDAR6. Large efforts have been channeled into various modulation mechanisms7. Microelectromechanical and nanoelectromechanical systems (MEMS and NEMS)8,9,10,11 have the advantages of low-cost and CMOS-compatibility, but the speed is limited up to MHz. Phase-change materials12,13,14 have fast, drastic, and non-volatile refractive index change, but lack continuous refractive index tuning and have a limited number of cycles constraining applicability to reconfigurable devices. Through molecule reorientation, liquid crystal can have index modulation over 10%, while under relatively low applied voltages Tunable liquid crystal metasurfaces, U.S. patent number 10,665,953 [Application Number 16/505,687]15. Techniques of liquid crystal integration have also advanced after decades of development. However, the tuning speeds are limited to kHz range16. Thermal-optic effects can induce relatively large refractive index changes17,18, but the speed is inherently limited and the on-chip thermal management can be challenging. The co-integration of transparent conductive oxide and metallic plasmonic structures5,6 has been demonstrated in epsilon-near-zero (ENZ) regime to control the wavefront of reflected light, but the low reflection amplitude induced by the optical loss of the materials and the ENZ regime is unavoidable.
In modern photonics, a multitude of technologies for tunable optics and frequency conversion19,20 are realized with nonlinear materials that have low loss and a strong χ effect, such as lithium niobate21,22, aluminum nitride23, and organic electro-optic (OEO) materials24. Their ultrafast responses make it possible to use RF or millimeter-wave control25. Developments in computational chemistry have also led to artificially engineered organic molecules that have record-high nonlinear coefficients with long-term and high-temperature stability26,27. However, their potential in modifying free-space light has been relatively unexplored until recently. Several OEO material-hybrid designs have demonstrated improved tunability of metasurfaces28,29,30. Utilizing dielectric resonant structures and RF-compatible coplanar waveguides, a free-space silicon-organic modulator has recently accomplished GHz modulation speed31. However, all demonstrations to date require high operating voltages ± 60V, due to low resonance tuning capability (frequency shift / voltage), which hinders their integration with electronic chips.
In this work, we propose combining high-Q metasurfaces based on slot-mode resonances with the unique nano-fabrication techniques enabled by OEO materials, which drastically reduces the operating voltage. The low voltage is mainly achieved from the ability to place the electrodes in close proximity to each other while hosting high-Q modes in between and the large overlap of the optical and RF fields in OEO materials. In the following sections, we first provide the design concepts and considerations for achieving a reduced operating voltage. Next, we numerically demonstrate the advantage of a particular selected mode compared to other supported modes in the structure. Finally, we experimentally realize our concepts and characterize the performance of the electro-optic metasurface.
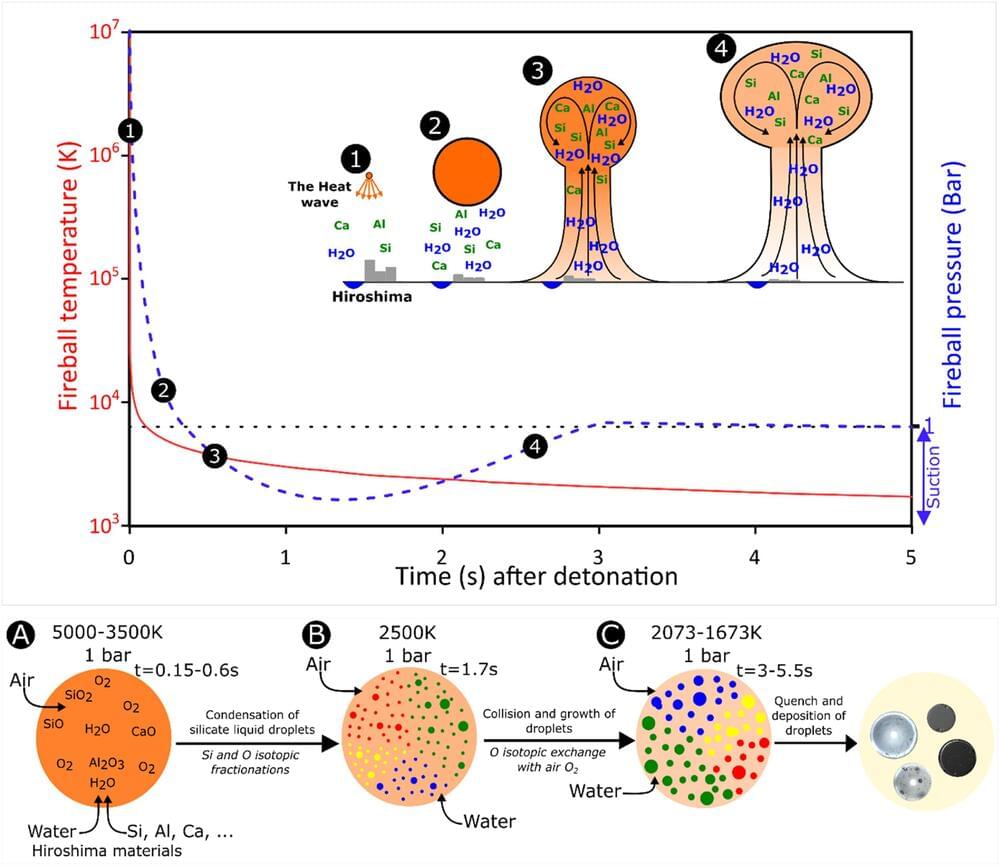
The atomic bombing of Hiroshima, Japan, by the United States in August 1945 was not only devastating at the time, resulting in the deaths of hundreds of thousands of people, but it has had long-standing impacts to the present day, particularly the elevated incidence of cancer from radiation.
Continued research of Hiroshima Bay has uncovered a new kind of debris from the fallout, known as Hiroshima glasses. These formed from vaporized materials of the bomb and the surrounding landscape and infrastructure being targeted.
New research published in Earth and Planetary Science Letters has analyzed the chemical and isotopic compositions of these glasses to ascertain their formation process during the nuclear event.

A chemical compound essential to all living things has been synthesized in a lab in conditions that could have occurred on early Earth, suggesting it played a role at the outset of life, finds a new study led by University College London researchers.
The compound, pantetheine, is the active fragment of Coenzyme A. It is important for metabolism—the chemical processes that maintain life. Earlier studies failed to synthesize pantetheine effectively, leading to suggestions that it was absent at life’s origin.
In the new study, published in the journal Science, the research team created the compound in water at room temperature using molecules formed from hydrogen cyanide, which was likely abundant on early Earth.
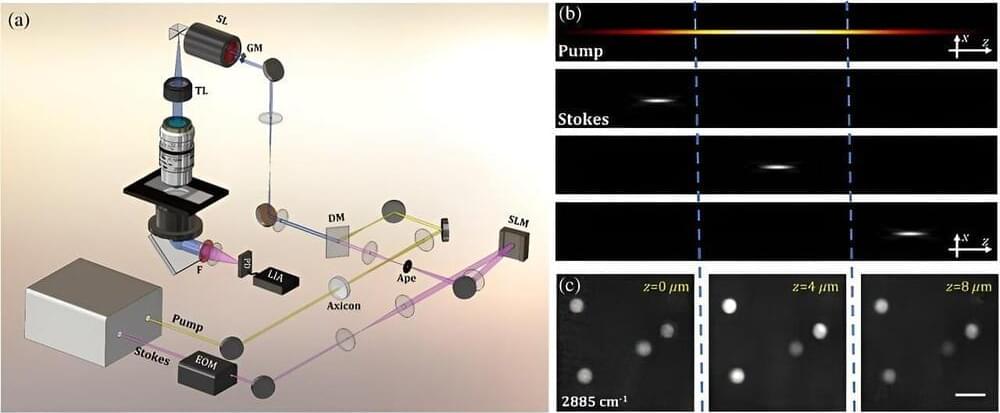
Understanding complex biological and biomedical systems is greatly aided by 3D imaging, which provides much more detailed information than traditional two-dimensional methods. However, live cell and tissue imaging remain challenging due to factors like limited imaging speed and significant scattering in turbid environments.
In this context, multimodal microscopy techniques are notable. Specifically, nonlinear techniques like CRS (coherent Raman scattering) use optical vibrational spectroscopy, providing precise chemical imaging in tissues and cells in a label-free way.
Furthermore, stimulated Raman scattering (SRS) microscopy, a CRS method, can accurately capture images of biomolecules due to the linear relationship between stimulated Raman intensity and the concentration of target molecules. It does so with high sensitivity and without interference from unwanted nonresonant backgrounds.

At approximately 18:17 CET (17:17 UTC) on Wednesday, February 21, 2024, ESA’s ERS-2 satellite completed its atmospheric reentry over the North Pacific Ocean. No damage to property has been reported.
ESA’s second European Remote Sensing satellite, ERS-2, was launched almost 30 years ago, on April 21, 1995. Together with the almost-identical ERS-1, it provided invaluable long-term data on Earth’s land surfaces, ocean temperatures, ozone layer, and polar ice extent that revolutionized our understanding of the Earth system. It was also called upon to monitor and assist the response to natural disasters.
“The ERS satellites have provided a stream of data which has changed our view of the world in which we live,” said ESA’s Director of Earth Observation Programmes, Simonetta Cheli. “They have provided us with new insights on our planet, the chemistry of our atmosphere, the behavior of our oceans, and the effects of mankind’s activity on our environment – creating new opportunities for scientific research and applications.”
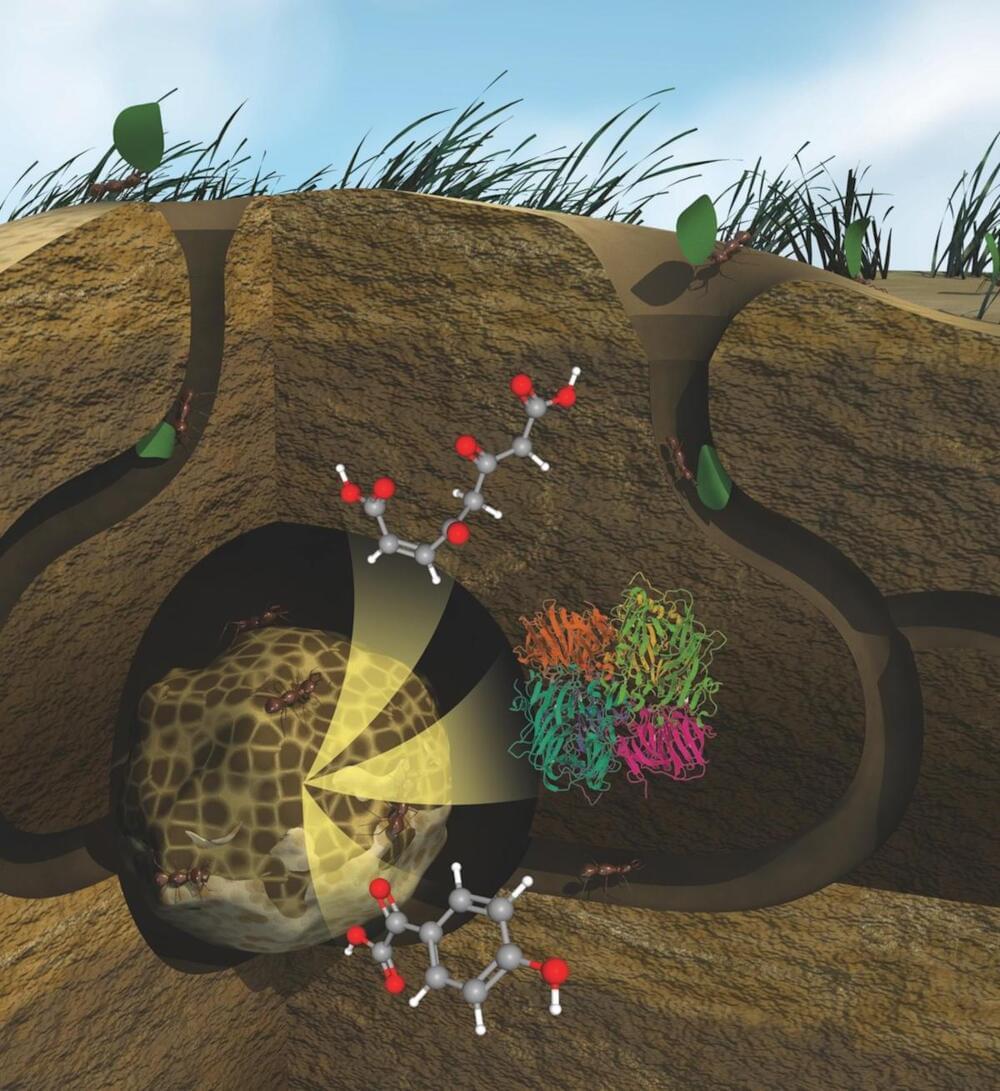
For years, researchers have dedicated themselves to developing methods that can effectively and economically break down plant materials, enabling their transformation into valuable bioproducts that enhance our daily lives.
Bio-based fuels, detergents, nutritional supplements, and even plastics are the result of this work. And while scientists have found ways to degrade plants to the extent needed to produce a range of products, certain polymers such as lignin, which is a primary ingredient in the cell wall of plants, remain incredibly difficult to affordably break down without adding pollutants back into the environment. These polymers can be left behind as waste products with no further use.
A specialized microbial community composed of fungus, leafcutter ants, and bacteria is known to naturally degrade plants, turning them into nutrients and other components that are absorbed and used by surrounding organisms and systems. But identifying all components and biochemical reactions needed for the process remained a significant challenge—until now.

In this first article in a series on philosophy and science, we take a look at materialism and why it is fundamental to science.
A short disclaimer before we read further: I’m a materialist. Materialism is a branch of philosophy to which the sciences, particularly the physical and life sciences, owe a lot. Materialism posits that the material world — matter — exists, and everything in the Universe, including consciousness, is made from or is a product of matter. An objective reality exists and we can understand it. Without materialism, physics, chemistry, and biology as we know it wouldn’t exist.
Another branch of philosophy, idealism, is in direct contradiction to materialism. Idealism states that, instead of matter, the mind and consciousness are fundamental to reality; that they are immaterial and therefore independent of the material world.
Injuries in the central nervous system heal poorly because cavities scar. Researchers hope to remedy this problem by filling the cavities in such a way that stem cells feel comfortable in them.
Researchers from Bochum and Dortmund have created an artificial cell environment that could promote the regeneration of nerves. Usually, injuries to the brain or spinal cord don’t heal easily due to the formation of fluid-filled cavities and scars that prevent tissue regeneration. One starting point for medical research is therefore to fill the cavities with a substance that offers neural stem cells optimal conditions for proliferation and differentiation. The team from Ruhr University Bochum and TU Dortmund University, both in Germany, showed that positively charged hydrogels can promote the survival and growth of stem cells.
Dr. Kristin Glotzbach and Professor Andreas Faissner from the Department of Cell Morphology and Molecular Neurobiology in Bochum cooperated with Professor Ralf Weberskirch and Dr. Nils Stamm from the Faculty of Chemistry and Chemical Biology at TU Dortmund University. The team describes the findings in the American Chemical Society Journal Biomaterials Science and Engineering from January 16, 2024.
