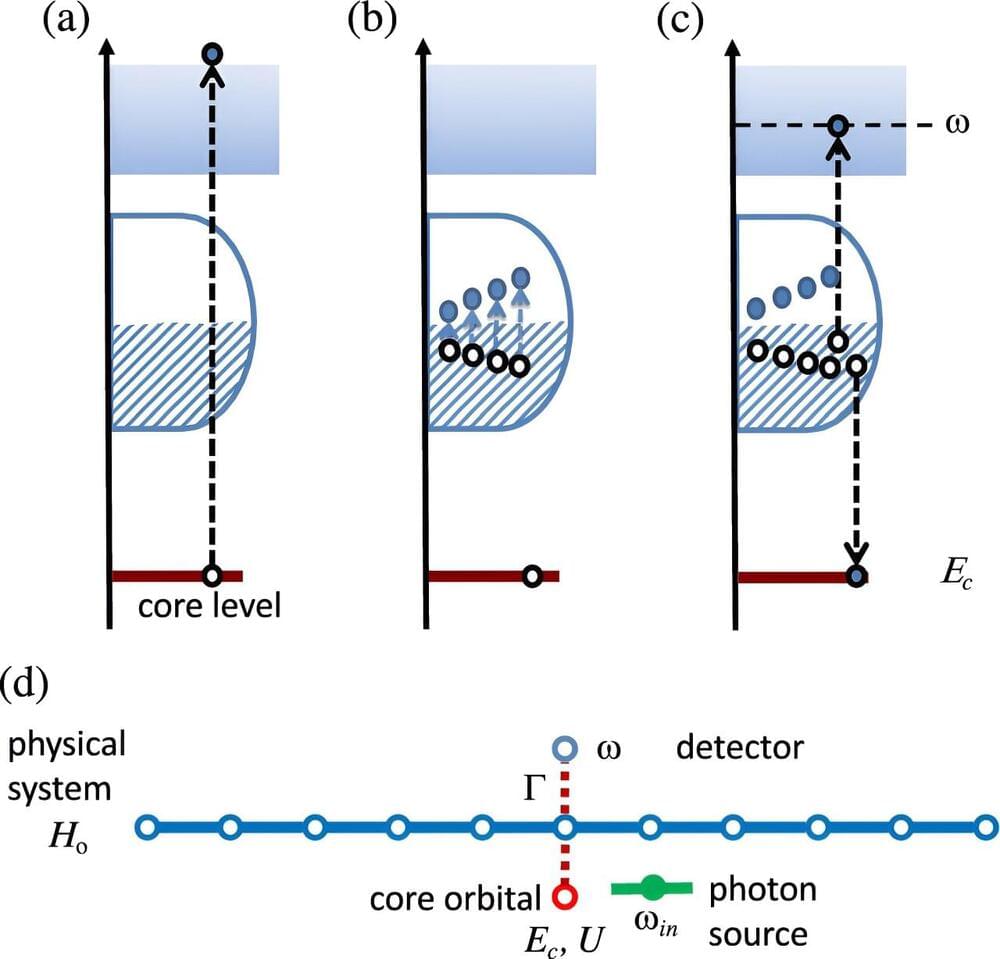
Auger electron spectroscopy (AES) is an incredibly useful technique for probing material samples—but current assumptions about the process ignore some of the key time-dependent effects it involves. So far, this has resulted in overly-simplified calculations, which have ultimately prevented the technique from reaching its full potential.
In a study published in The European Physical Journal Plus Alberto Noccera at the University of British Columbia, Canada, together with Adrian Feiguin at Northeastern University, United States, developed a new computational approach which offers a more precise theoretical description of the AES process, while taking its time dependence into account. Their method could help researchers to improve their quality of material analysis across a wide array of fields: including chemistry, environmental science, and microelectronics.
In the Auger process, an inner-shell electron is initially kicked out of its atom, often through an impact with an energetic light pulse. Afterward, the vacancy it leaves behind is filled by an outer-shell electron.