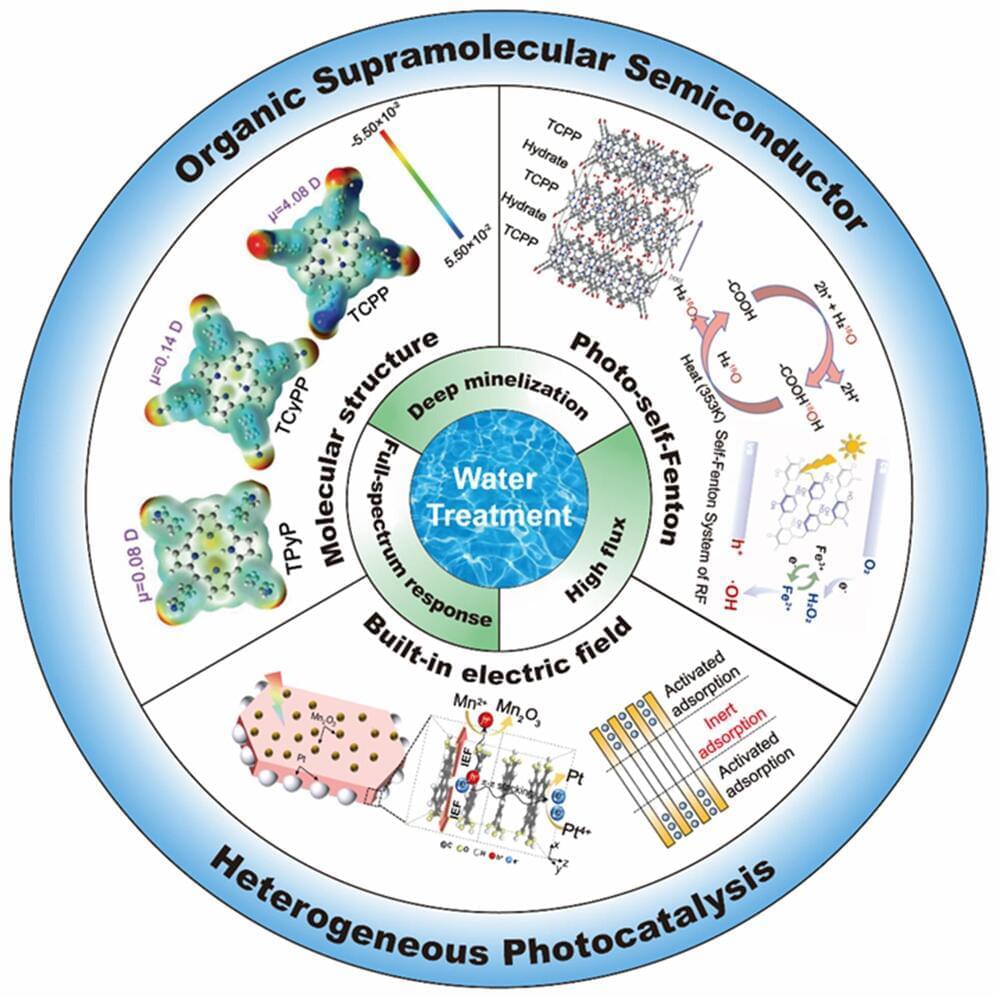
Refractory organic pollutants, including phenols, perfluorinated compounds, and antibiotics, are abundant in various industrial wastewater streams such as chemical, pharmaceutical, coking, and dyeing sectors, as well as municipal and domestic sources. These pollutants pose significant threats to ecological well-being and human health.
The imperative to achieve complete removal of organic contaminants from water and facilitate water recycling is paramount for enhancing environmental quality and ensuring sustainable economic and social progress. Addressing the efficient removal of recalcitrant organic pollutants in water is not only a focal point in environmental chemical pollution control research but also a pivotal technical challenge constraining industrial wastewater reuse.
Advanced oxidation processes (AOPs), especially heterogeneous AOPs, yield strongly reactive oxygen species including ·OH, ·O2-, and ·SO4- to oxidize organic pollutants under ambient conditions, are appealing wastewater treatment technologies for decentralized systems. AOPs often need excessive energy input (UV light or electricity) to activate soluble oxidants (H2O2, O3, persulfates), thus more cost-effective AOPs are urgently required.