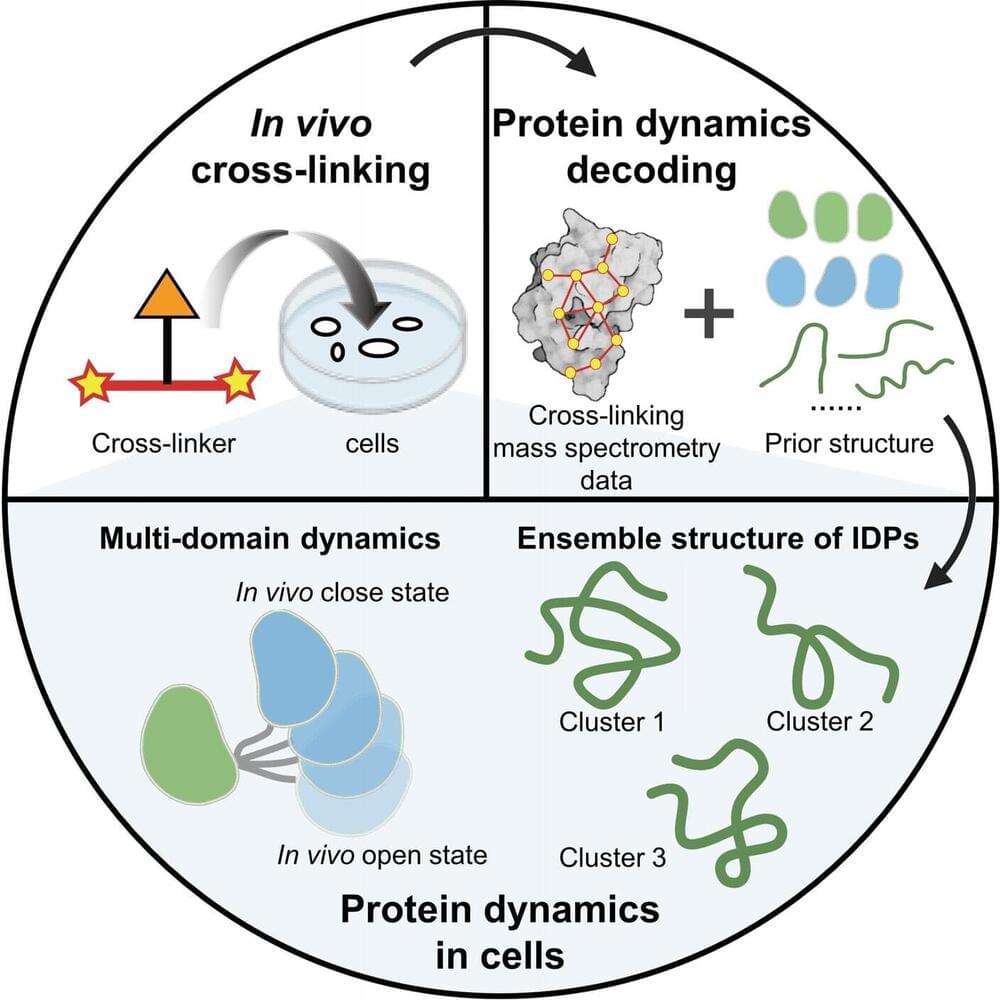
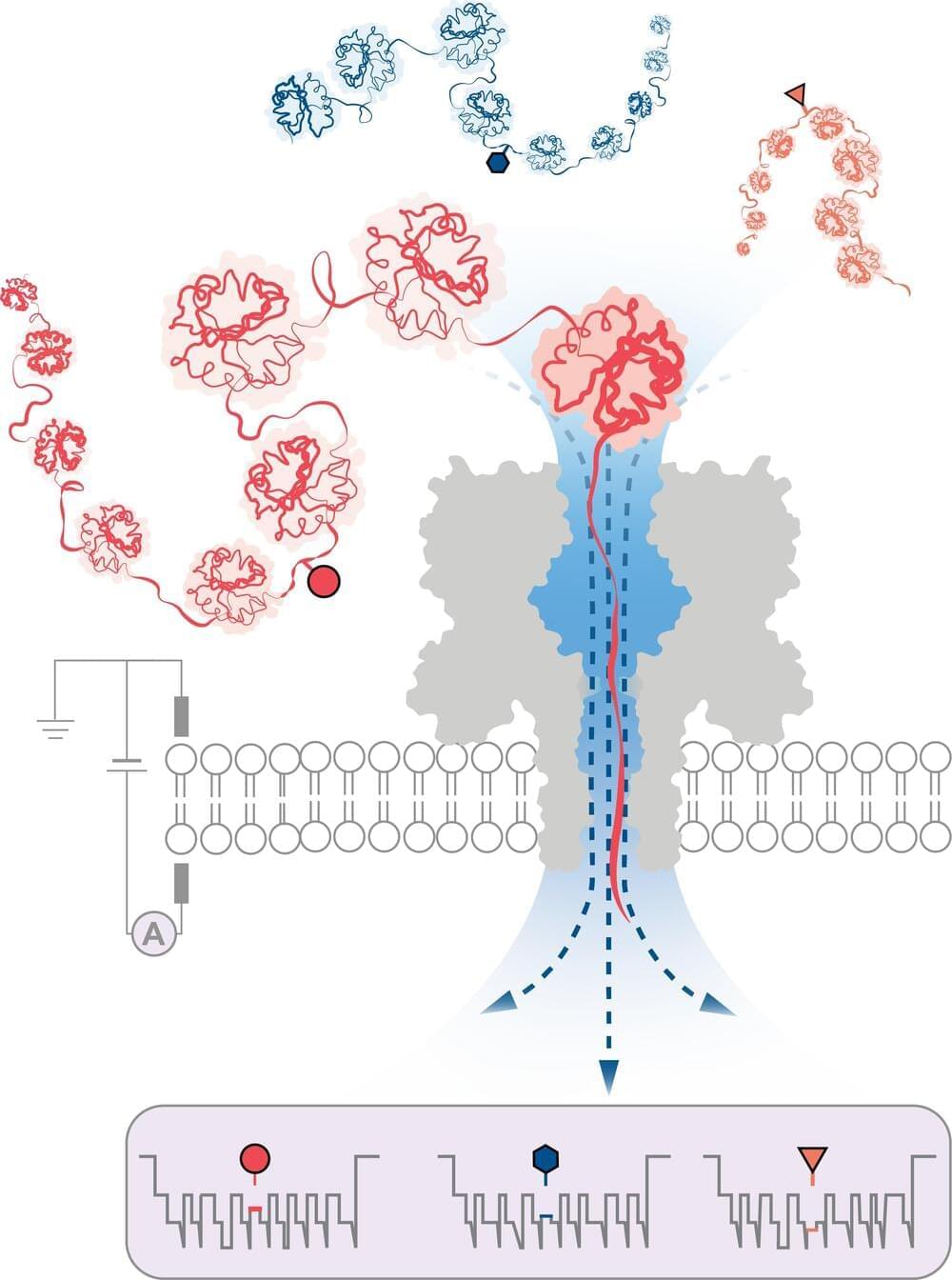
Protein dynamics play a crucial role in diverse functions. The intracellular environment significantly influences protein dynamics, particularly for intrinsically disordered proteins (IDPs).
A research group led Prof. Zhang Lihua from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS), in collaboration with Assoc. Prof. Gong Zhou from the Precision Measurement Science and Technology Innovation Research Institute of CAS, has proposed a strategy using in-vivo chemical cross-linking and mass spectrometry (in-vivo XL-MS) to decode the dynamic structure of proteins within cells.
In-vivo XL-MS is potential for analyzing the dynamic structure of proteins within cells due to its high throughput, high sensitivity, and low requirements for protein purity.