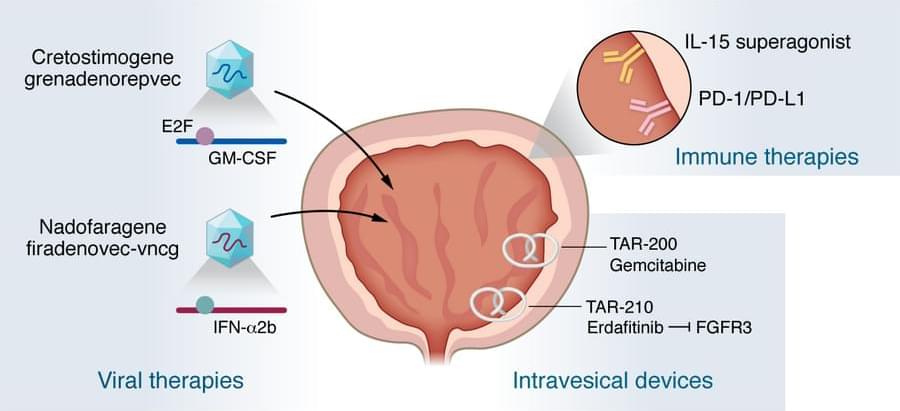
Cancer is characterized by the uncontrolled cell proliferation, invasion, and check-point evasion of abnormal cells that are mostly nonfunctional. Cancer can arise due to diet insufficiencies, inherited mutations, and tobacco consumption, to say the least [1, 2]. Cancer’s incident is increasing due to the sedentary lifestyle, overpopulated, polluted megacities, and individuals’ growing desire for consuming processed foods containing preservatives additives [3], [4], [5]. Since cancers might not manifest symptoms in their early onset, it would be difficult or even improbable to treat them when they are diagnosed in their late stage. By and large, tumors are composed of two main parts, including the proliferating cells and stroma, which contains connective tissue and blood supply [6]. Chemotherapy has been among our best options against malignancies.
Chemotherapy is defined by the administration of numerous drugs and chemicals either alone or in combination to kill the cancer cells. Chemotherapeutic drugs kill cancer cells or control their progression all over the patient’s body, while radiation-and surgery-based treatments are directed to a particular site. Cure, control, and palliation are the three objectives of chemotherapies. Killing cancer cells by implementing chemotherapy drugs means “Cure”, whereas “Control” defines the situation that full remission seems far-fetched; therefore, the objective of the therapy would be to decrease the tumor size or to diminish the growth rate and angiogenesis. Palliation aims to alleviate the pain, symptoms, and medical conditions arisen due to cancer. It is mostly accomplished when cancer is in the advanced stages and cannot be eradicated; therefore, our aim would be to improve the quality of life.
The chemotherapy prescription approaches rely on various elements, including the cancer’s type, the cancer’s stage, the patient’s age, the patient’s general health status, the other concurrent health issues, and the history of receiving chemotherapies. Since chemotherapeutic drugs cannot distinguish normal cells against cancerous cells, the prescribed dosage is the other crucial aspect toward achieving the best possible response. The administration dosage depends on the patient’s weight, body surface area, age, nutrition status, history of radiation therapy, and blood cell count. Besides, a suitable drug administration schedule might help obtain the most efficient anti-cancer activity and minimum side effects [7, 8].