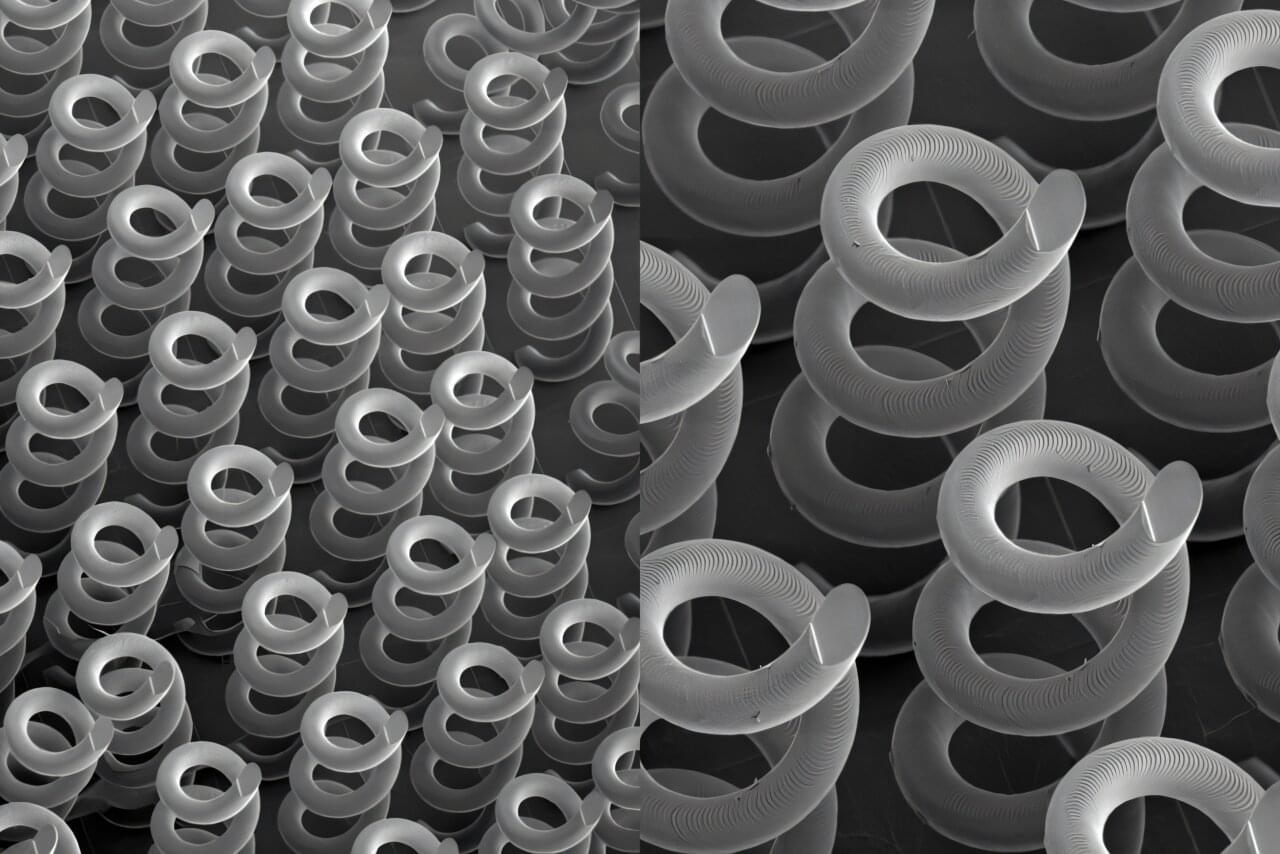
Researchers at Lawrence Livermore National Laboratory (LLNL) have optimized and 3D-printed helix structures as optical materials for terahertz (THz) frequencies, a potential way to address a technology gap for next-generation telecommunications, non-destructive evaluation, chemical/biological sensing and more.
The printed microscale helices reliably create circularly polarized beams in the THz range and, when arranged in patterned arrays, can function as a new type of Quick Response (QR) for advanced encryption/decryption. Their results, published in Advanced Science, represent the first full parametric analysis of helical structures for THz frequencies and show the potential of 3D printing for fabricating THz devices.