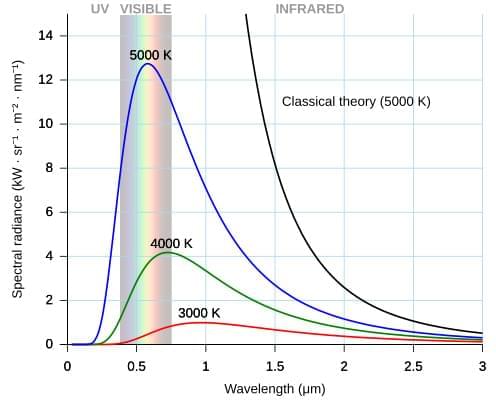
Chemists from the University of Warwick and Monash University have discovered a promising new antibiotic that shows activity against drug-resistant bacterial pathogens, including MRSA and VRE.
Antimicrobial resistance (AMR) is one of the world’s most urgent health challenges, with the WHO’s new report showing there are ‘too few antibacterials in the pipeline’. Most of the ‘low-hanging fruit’ has already been found, and the limited commercial incentives deter investment in antibiotic discovery.
In a new study published in the Journal of the American Chemical Society, researchers from the Monash Warwick Alliance Combatting Emerging Superbug Threats Initiative have discovered a promising new antibiotic — pre-methylenomycin C lactone. The newly discovered antibiotic was ‘hiding in plain sight’ – as an intermediate chemical in the natural process that produces the well-known antibiotic methylenomycin A.