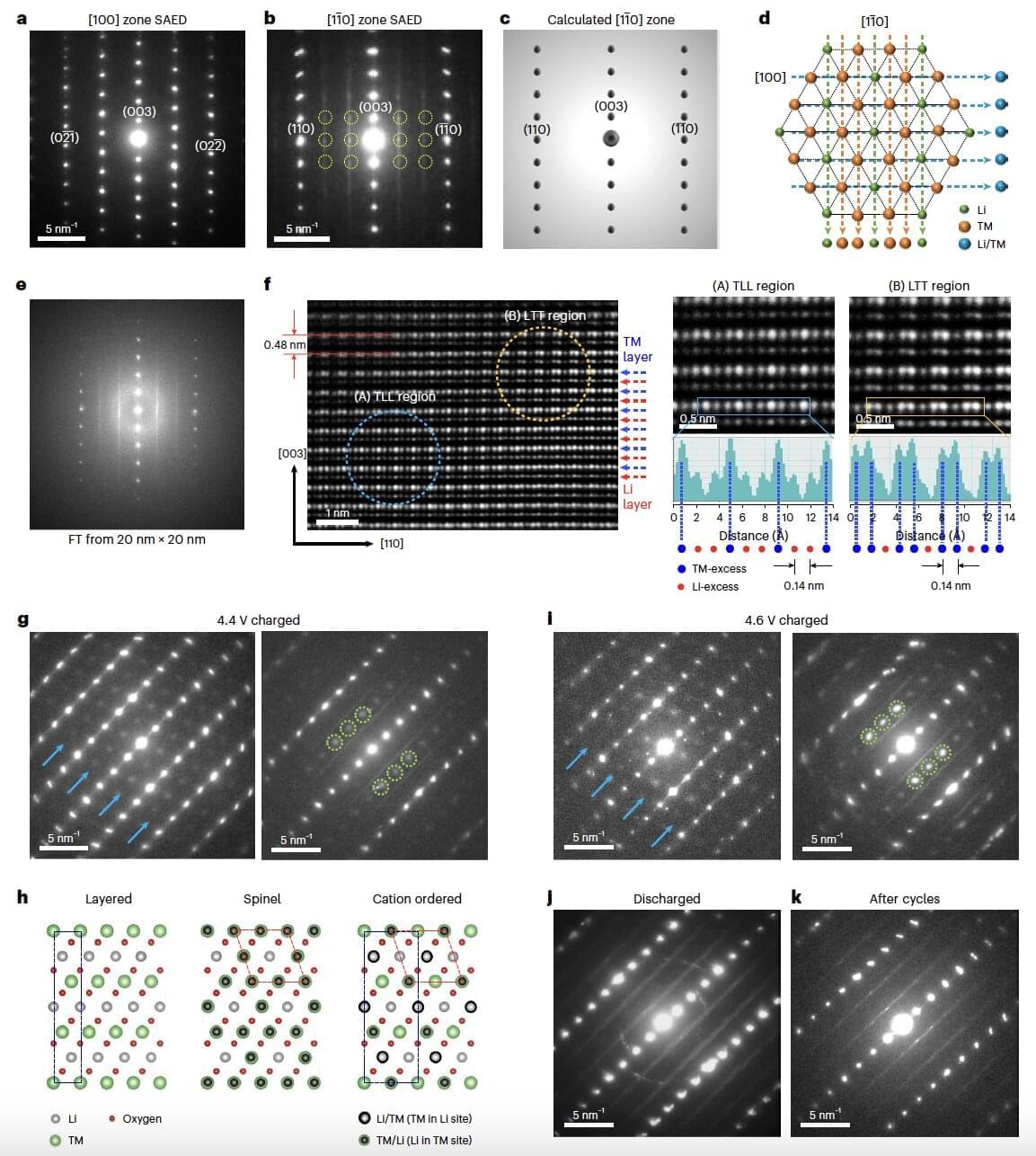
Researchers at University of Limerick (UL) have developed a battery that could reshape the future of electric vehicles and portable electronics. Their breakthrough in energy storage technology has seen the development of the world’s first full-cell dual-cation battery.
This innovative system combines lithium and sodium ions to significantly enhance both battery capacity and stability, marking a new frontier in sustainable energy research.
The work, published in Nano Energy, was led by Hugh Geaney, Associate Professor of Chemistry at UL’s Department of Chemical Sciences and Principal Investigator at UL’s Bernal Institute, and Government of Ireland postdoctoral fellow, Dr. Syed Abdul Ahad, his colleague at the Department and the Bernal Institute.