Forecasters are warning that a new storm could bring ice and power outages across the South this weekend.


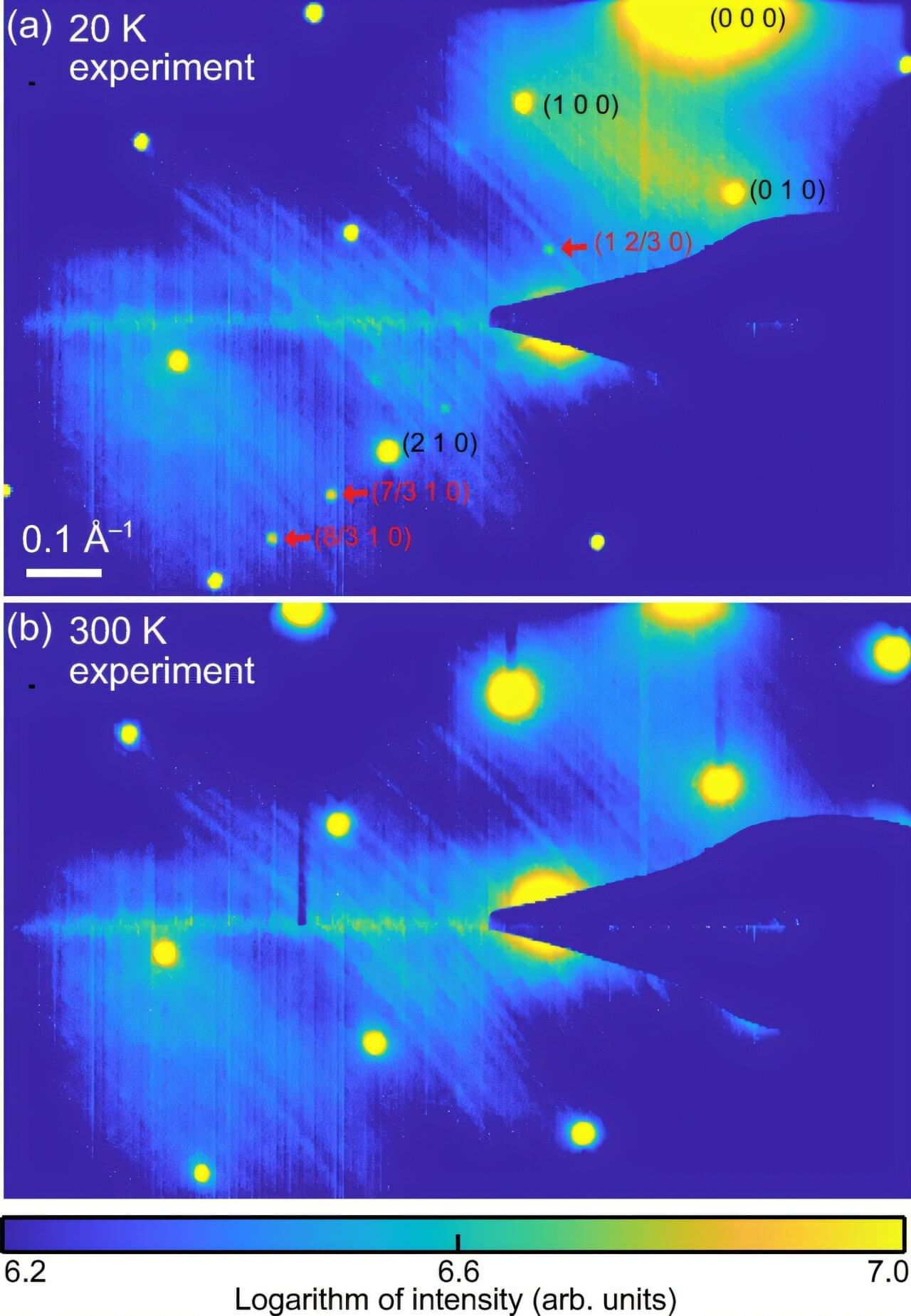
The mystery of quantum phenomena inside materials—such as superconductivity, where electric current flows without energy loss—lies in when electrons move together and when they break apart. KAIST researchers have succeeded in directly observing the moments when electrons form and dissolve ordered patterns.
Research teams led by Professors Yongsoo Yang, SungBin Lee, Heejun Yang, and Yeongkwan Kim of the Department of Physics, in an international collaboration with Stanford University, have become the first in the world to spatially visualize the formation and disappearance of charge density waves (CDWs) inside quantum materials.
The research is published in Physical Review Letters.

Exciting electronic characteristics emerge when scientists stack 2D materials on top of each other and give the top layer a little twist.
The twist turns a normal material into a patterned lattice and changes the quantum behavior of the material. These twisted materials have shown superconductivity—where a material can conduct electricity without energy loss—as well as special quantum effects. Researchers hope these “twistronics” could become components in future quantum devices.
But creating these extremely thin stacked structures, called moiré superlattices, is difficult to do. Scientists usually peel off single layers of material using Scotch tape and then carefully stick those layers together. However, the method has a very low success rate, often leaves behind contamination between layers and produces tiny samples smaller than the width of a human hair.
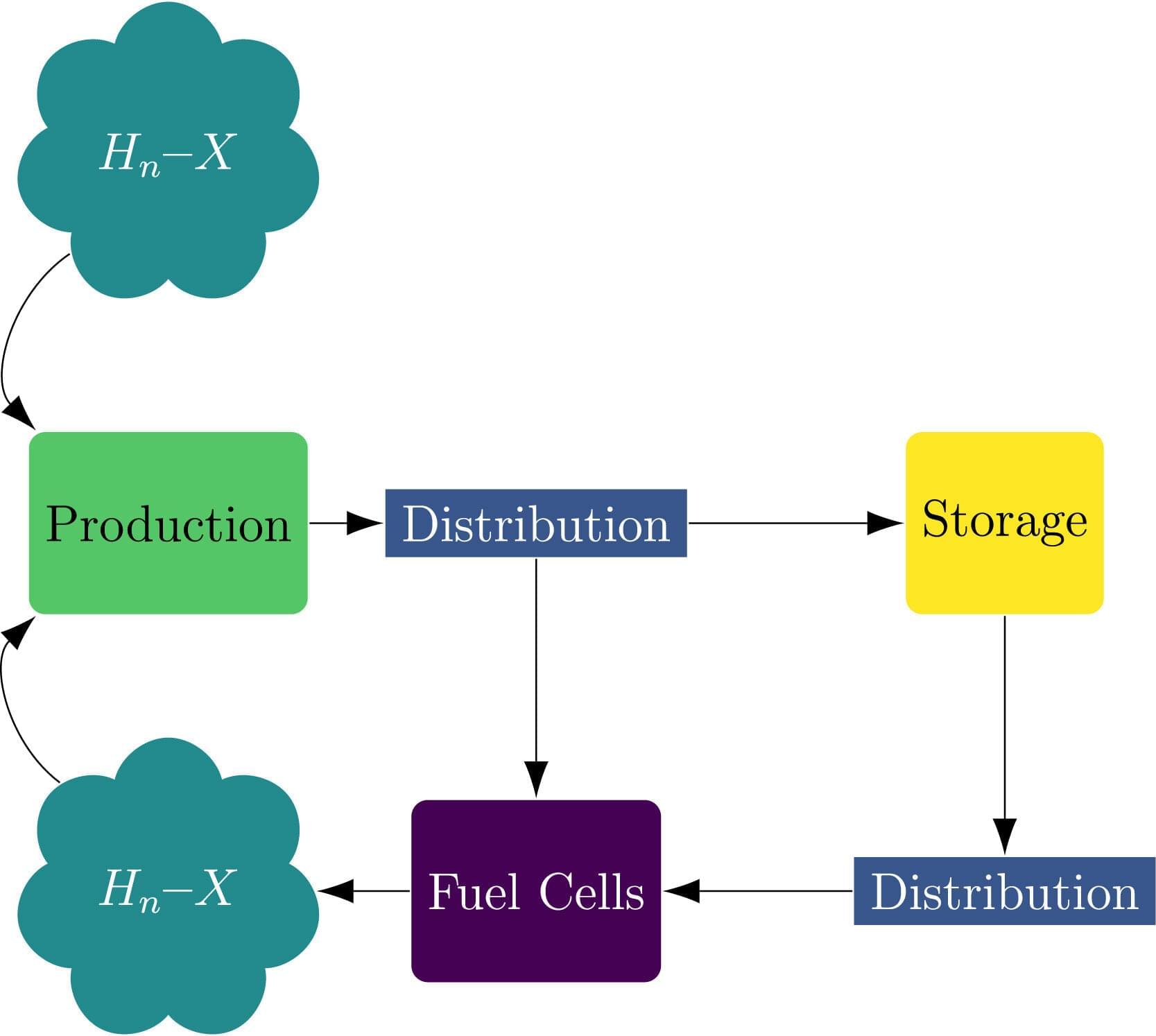
A study from Edinburgh Business School at Heriot-Watt University found that while hydrogen production, storage and fuel cell technologies are advancing rapidly, the hydrogen distribution infrastructure is developing at half the speed, creating a critical bottleneck that could put billions in clean energy at risk.
The paper “Dynamics of knowledge production: A relational-event analysis of patent citation hazards in hydrogen technologies” was published in Sustainable Futures.
The findings are an important milestone in recognizing that, while other hydrogen technologies improve and costs fall, distribution expenses could take up a large share of hydrogen system budgets, significantly limiting overall efficiency and growth of the hydrogen sector.
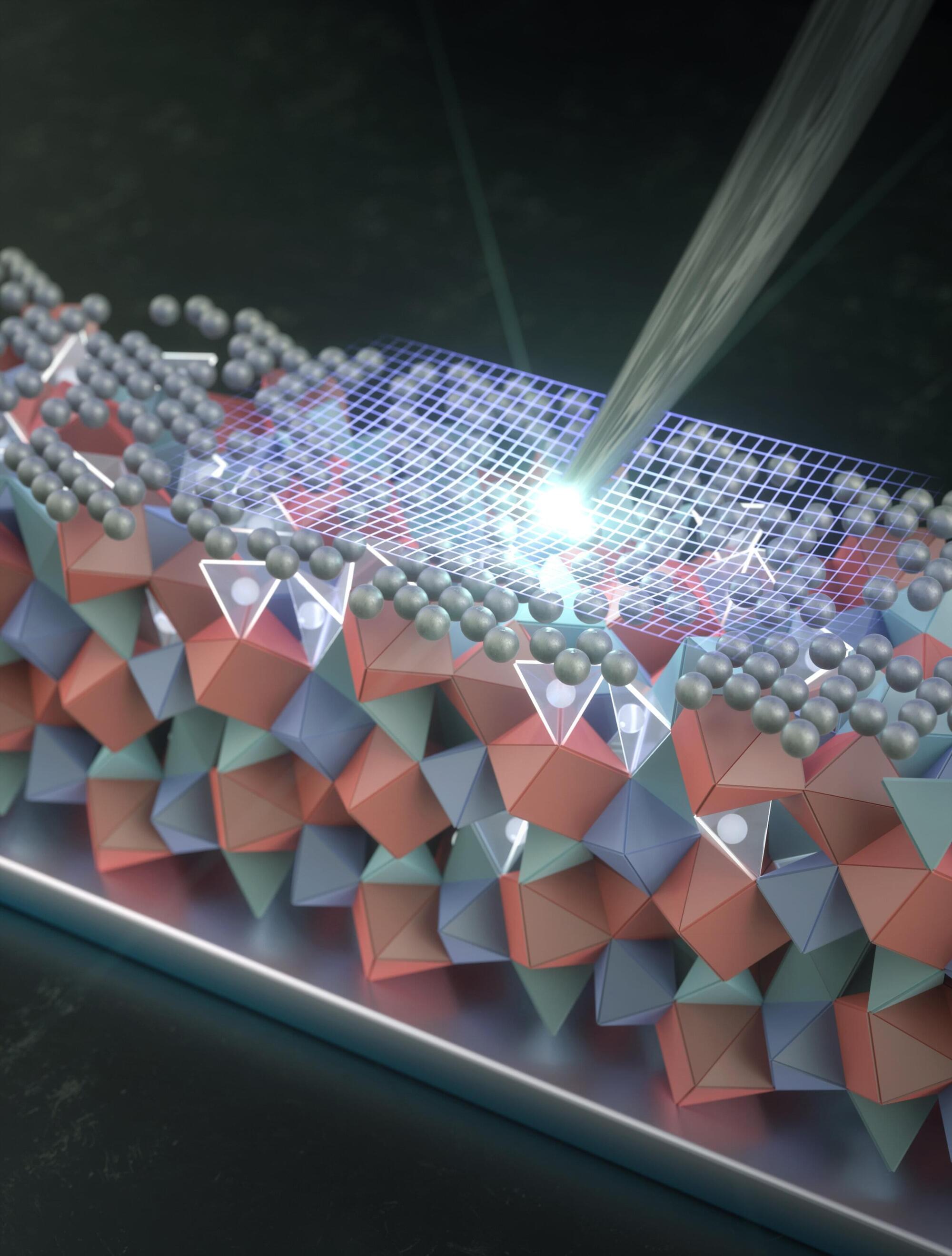
A solid—rather than liquid—electrolyte between the opposite electrodes of a battery should, in theory, enable a rechargeable lithium metal battery that is safer, packs much more energy, and charges considerably faster than the lithium-ion batteries commercially available today.
For decades, scientists and engineers have explored several paths to realize the great promise of lithium-metal batteries. A major problem with the solid, crystalline electrolytes under study has been the formation of microscopic cracks that grow during use until the battery fails.
Stanford researchers, building on findings they published in 2023 that identified how these tiny fractures, dents, and other imperfections form and expand, have discovered that annealing an extremely thin silver coating on the solid electrolyte’s surface seems to largely solve the problem.
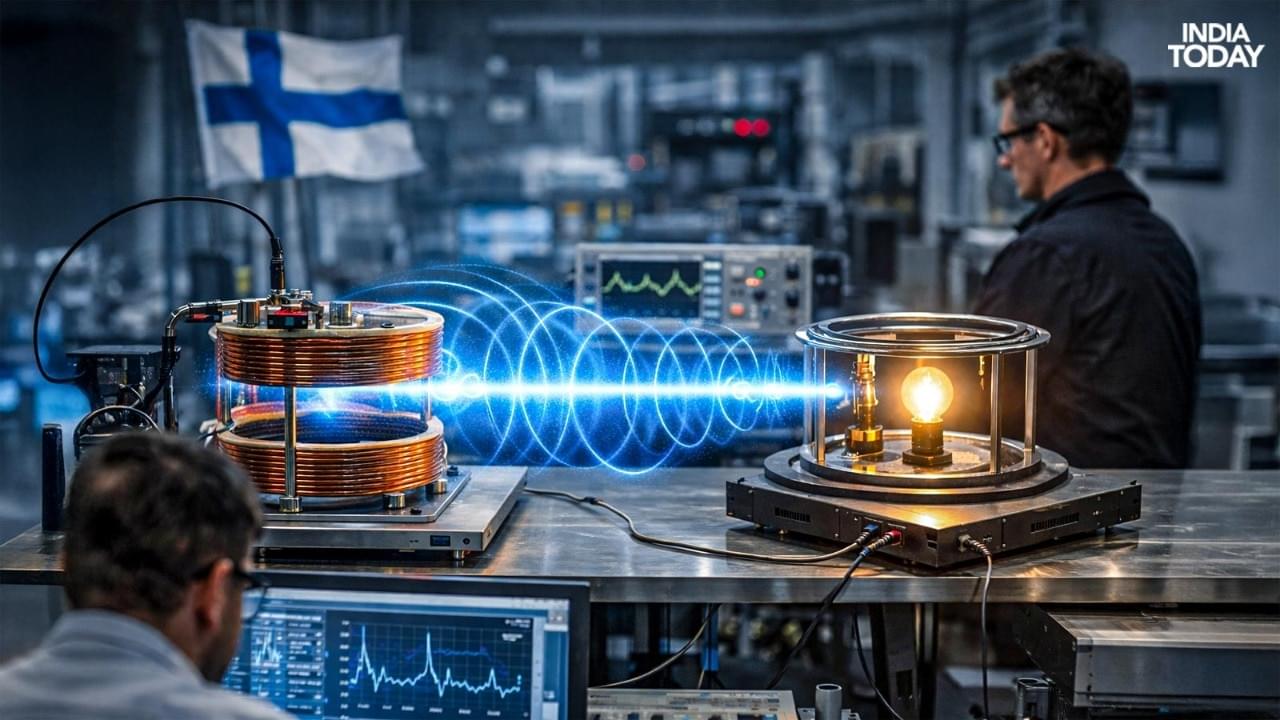
In controlled experiments, engineers have shown that electricity can be transmitted through the air using highly controlled electromagnetic fields and resonant coupling techniques, conceptually similar to the way data is sent via Wi-Fi but tailored for energy transfer.
These approaches build on decades of research into magnetic resonance and inductive power transfer, which seek to send energy efficiently across short distances without physical contact between transmitter and receiver…
…Past research from the university demonstrated that magnetic loop antennas can transfer power wirelessly at relatively high efficiency over short ranges, offering insights into how to optimize coupling and reduce energy losses.
More recent demonstrations reported in global tech news describe Finnish teams successfully powering small devices through the air using wireless power transfer methods.
Finland continues to make progress in the field of wireless electricity transmission, an area of research that aims to send power through the air without the use of traditional cables or plugs.
Recent demonstrations and experiments by Finnish researchers have highlighted steady advancements in technology that could one day reshape how devices are powered, though widespread commercial deployment remains distant.
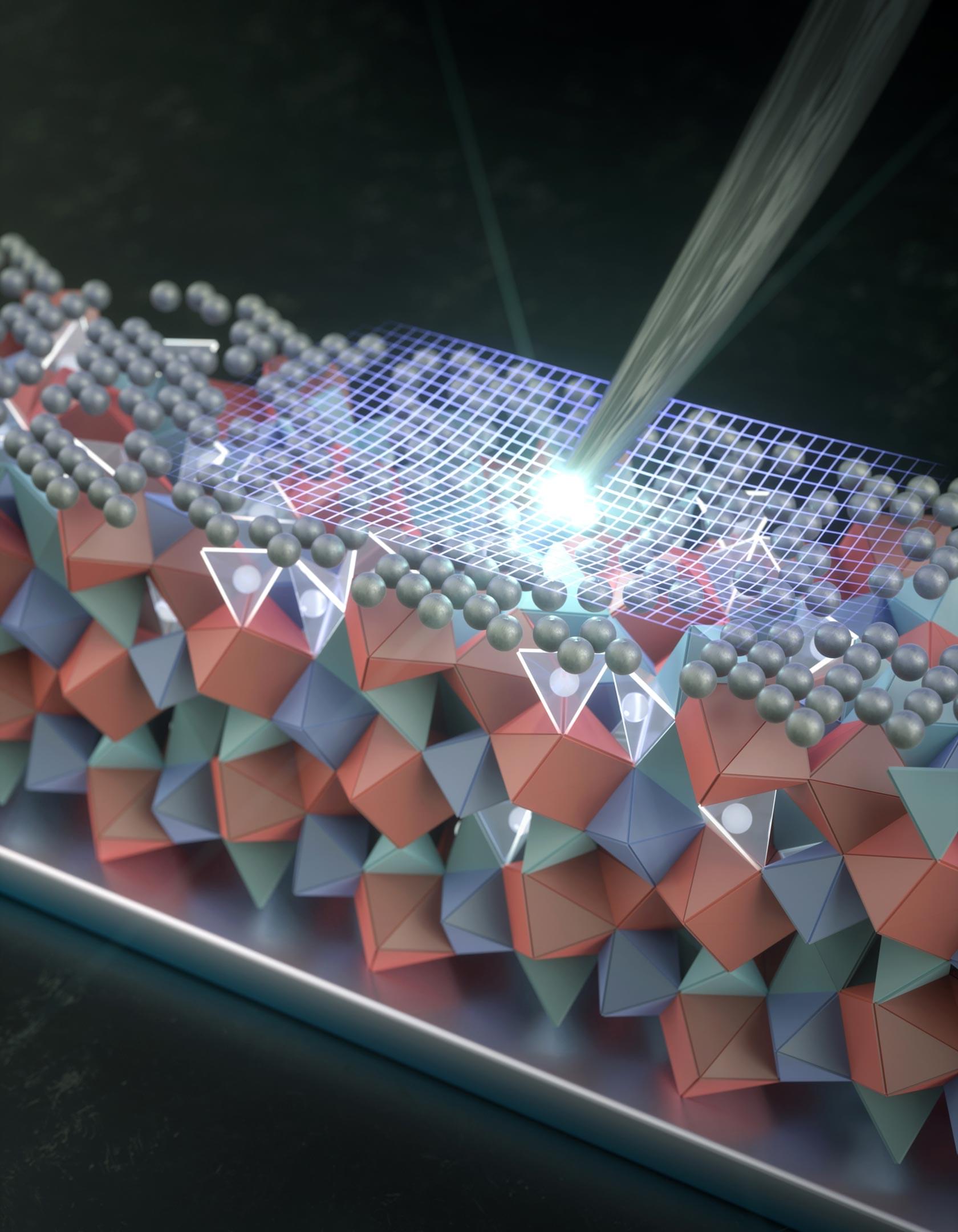
A nanoscale silver coating could be the key to making ultra-powerful solid-state batteries finally work.
Replacing the liquid electrolyte inside today’s batteries with a solid one could unlock a new generation of rechargeable lithium metal batteries. In theory, these batteries would be safer, store far more energy, and recharge much faster than the lithium-ion batteries now in widespread use. Scientists and engineers have been chasing this goal for decades, but progress has been slowed by a persistent flaw. Solid, crystal-based electrolytes tend to develop microscopic cracks that gradually spread during repeated charging and use, eventually causing the battery to fail.
A thin silver layer with a big impact.
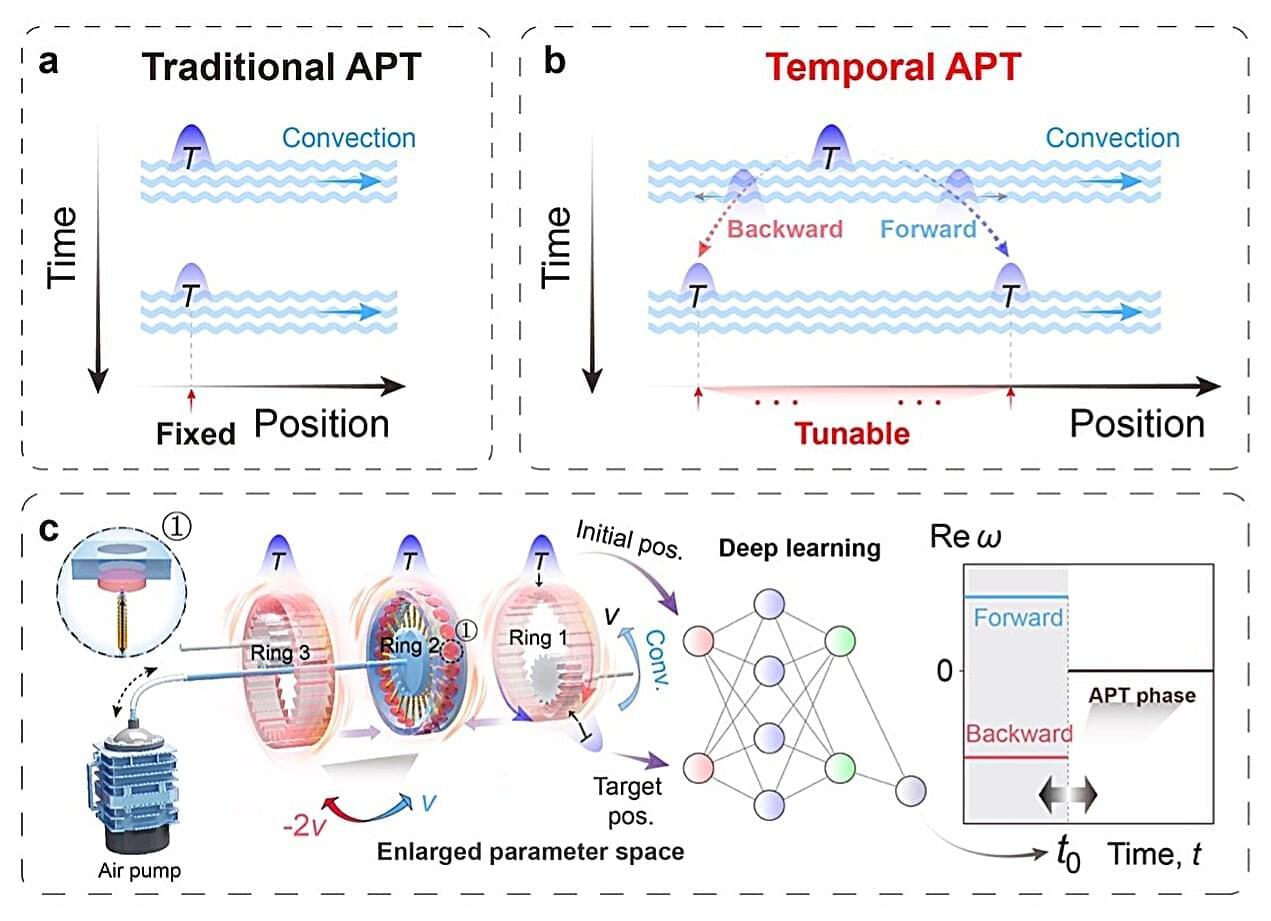
The movement of waves, patterns that carry sound, light or heat, through materials has been widely studied by physicists, as it has implications for the development of numerous modern technologies. In several materials, the movement of waves depends on a physical property known as parity-time (PT) symmetry, which combines mirror-like spatial symmetry with a symmetry in a system’s behavior when time runs forward and backwards.
Systems with PT symmetry can suddenly alter their behavior when they pass specific thresholds known as phase transitions, where they shift from balanced to unbalanced states. So far, systems exhibiting PT symmetry are mostly static, meaning that they exhibit fixed properties over time.
In Nature Physics, researchers at University of Shanghai for Science and Technology, Fudan University and National University of Singapore introduce a new concept called temporal anti-parity–time (APT) symmetry, which delineates more clearly both where and when a phase transition happens in a non-Hermitian system, a system that exchanges energy with its surroundings.

Earth’s oceans reached their highest heat levels on record in 2025, absorbing vast amounts of excess energy from the atmosphere. This steady buildup has accelerated since the 1990s and is now driving stronger storms, heavier rainfall, and rising sea levels. While surface temperatures fluctuate year to year, the ocean’s long-term warming trend shows no sign of slowing.