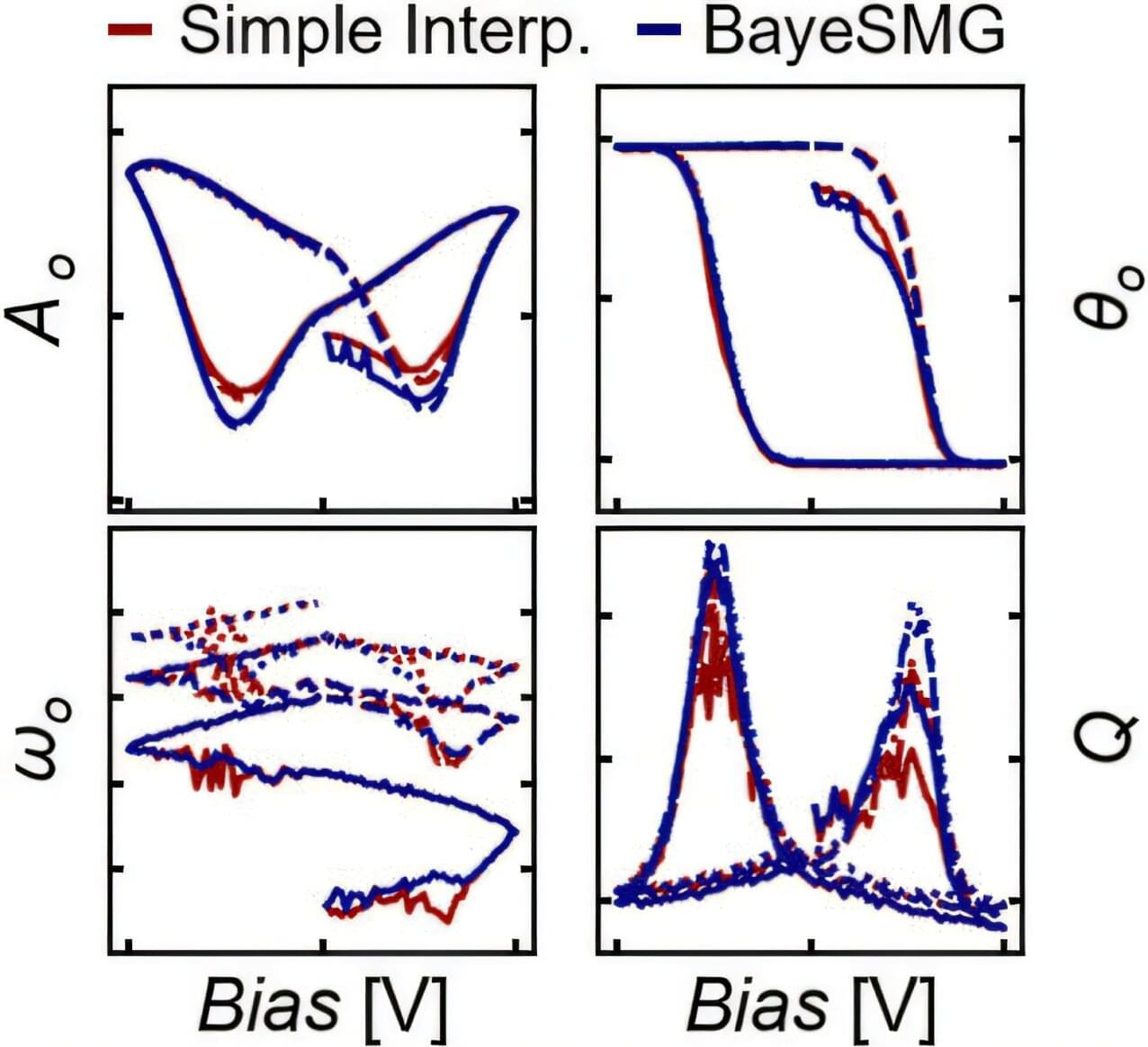
In the world of nanotechnology, seeing clearly isn’t easy. It’s even harder when you’re trying to understand how a material’s properties relate to its structure at the nanoscale. Tools like piezoresponse force microscopy (PFM) help scientists peer into the nanoscale functionality of materials, revealing how they respond to electric fields. But those signals are often buried in noise, especially in instances where the most interesting physics happens.
Now, researchers at Georgia Tech have developed a powerful new method to extract meaningful information from even the noisiest data, or when, alternatively, the response of the material is the smallest. Their approach, which combines physical modeling with advanced statistical reconstruction, could significantly improve the accuracy and confidence of nanoscale measurement properties.
The team’s findings, led by Nazanin Bassiri-Gharb, Harris Saunders, Jr. Chair and Professor in the George W. Woodruff School of Mechanical Engineering and School of Materials Science and Engineering (MSE), are reported in Small Methods.