“Of all currently known temperate exoplanets, LHS 1,140 b could well be our best bet to one day indirectly confirm liquid water on the surface of an alien world beyond our Solar System,” said Charles Cadieux.
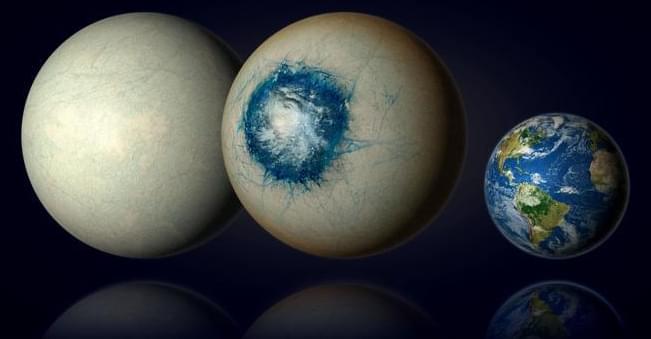
The search for Earth’s twin just got a little closer as astronomers recently presented findings regarding a potential icy or watery “super-Earth” called LHS 1,140 b, which is located approximately 49 light-years from Earth and whose radius is approximately 1.7 times our planet, along with orbiting within its star’s habitable zone. What makes this finding unique is LHS 1,140 b was previously hypothesized to be a mini-Neptune and astronomers speculate could be completely covered in either ice or water.
The findings were recently accepted to The Astrophysical Journal Letters and hold the potential to help astronomers better understand the formation and evolution of exoplanets, and specifically Earth-sized exoplanets within their star’s habitable zone.