Transposable elements in cancer therapy.
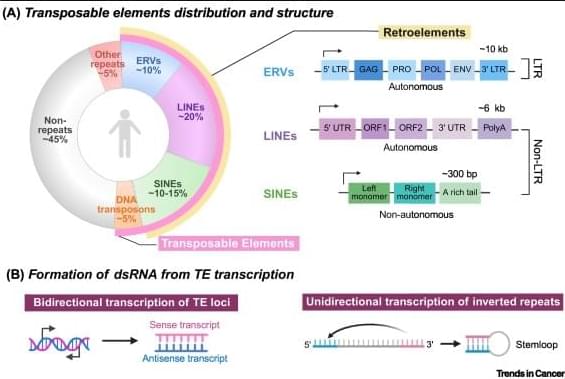
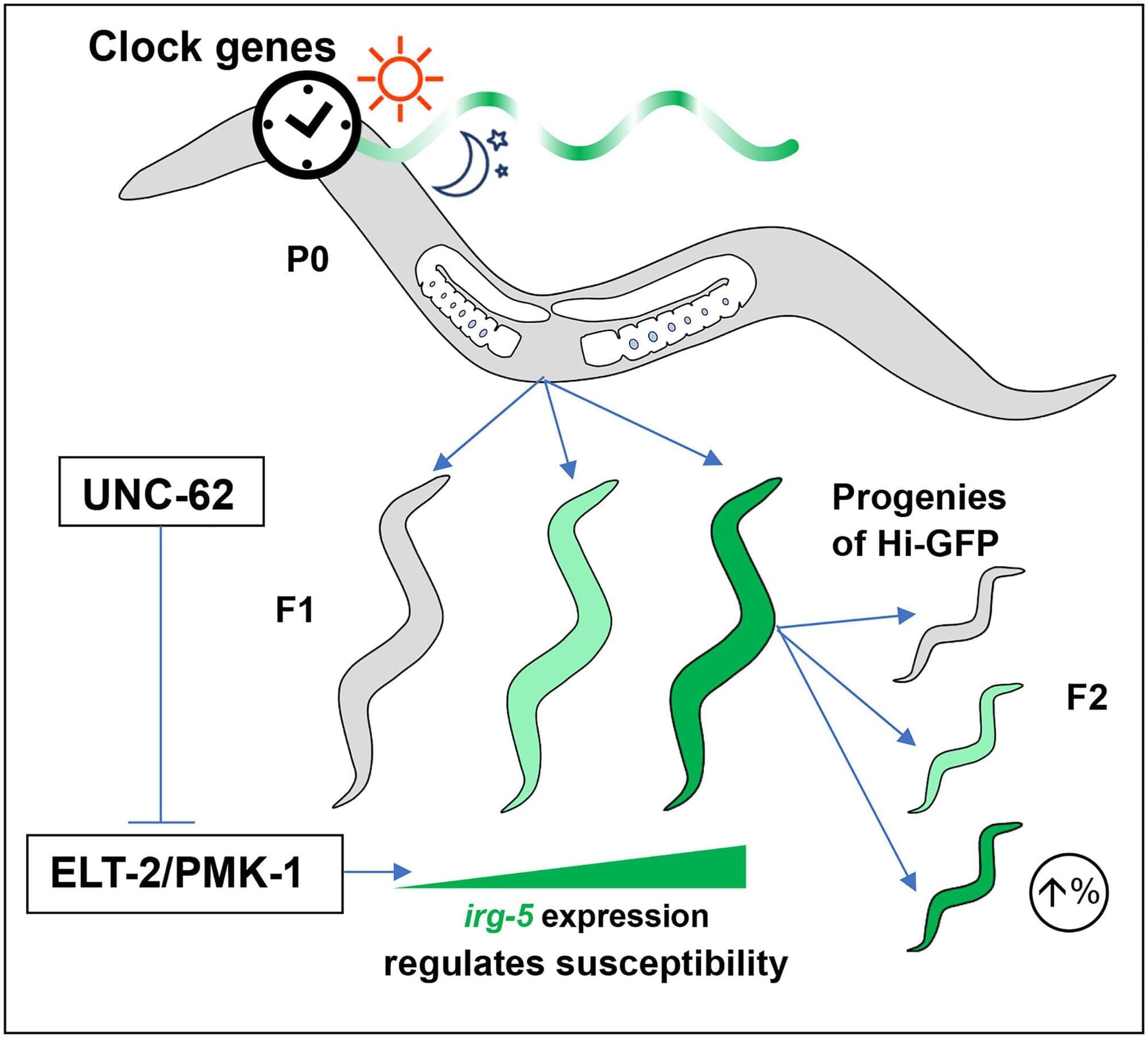
Transposable elements (TEs) are a major source of immunogenic nucleic acids that can be therapeutically reactivated in cancer cells to induce a state of viral mimicry.
TE expression can trigger innate immune sensing pathways, including type I interferon responses, and promote immunogenic cell death via sensors such as RIGI, MDA5, cGAS, and Z-DNA binding protein 1.
Although initially described in the context of epigenetic therapies, viral mimicry is now recognized as a shared response to diverse cancer treatment modalities, including chemotherapies and targeted therapies.
Despite their distinct primary mechanisms, these treatments converge on TE reactivation through disruption of DNA/histone methylation, p53 activation, and perturbation of mRNA splicing.
Therapeutic resistance to chemotherapy, radiation, and targeted agents is associated with TE silencing, identifying TE repression as a targetable axis of resistance.
Combination strategies to induce immunogenic TE expression can further enhance viral mimicry and boost antitumor immunity. https://sciencemission.com/Viral-mimicry-in-cancer-therapy