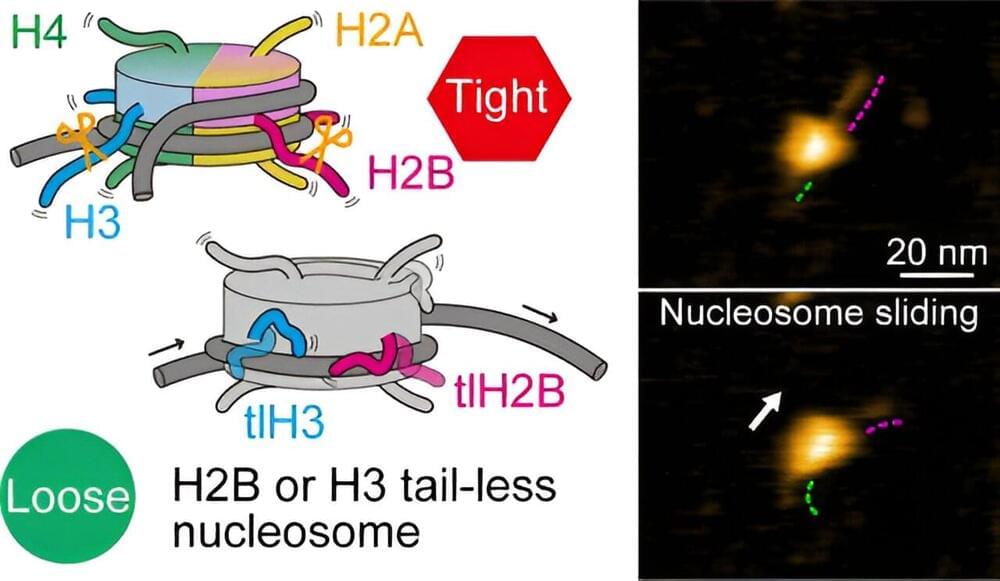
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhDDiscount Links: Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7x…
Category: genetics – Page 147

Tuberous Sclerosis
Tuberous sclerosis is a rare genetic disease that causes benign tumors to grow in the brain and other organs. The disease can be mild, or it can cause severe disabilities. Tuberous sclerosis has no cure, but treatments can help symptoms. More info here.
Tuberous sclerosis (TSC) is a rare genetic disease. It causes benign tumors in the brain and other organs. Learn about symptoms and what can help.

Biology beyond the genome | Denis Noble
Denis Noble discusses common misconceptions in genetics. Are our genes really as deterministic as we think they are?Watch the full talk] at https://iai.tv/vid…

Ed Boyden: Let’s Bring Engineers into Studying the Brain
Subscribe: RSS
I met Prof. Ed Boyden at last year’s Global Future 2045 conference in New York. There I was highly impressed with Boyden’s impressive work in neuroscience in general and optogenetics in particular, as well as the profound implications it would have on our ability to understand and manipulate the brain. And so I knew instantly I must bring him for an interview.

How Quantum Computers Could Illuminate the Full Range of Human Genetic Diversity
Genomics is revolutionizing medicine and science, but current approaches still struggle to capture the breadth of human genetic diversity. Pangenomes that incorporate many people’s DNA could be the answer, and a new project thinks quantum computers will be a key enabler.
When the Human Genome Project published its first reference genome in 2001, it was based on DNA from just a handful of humans. While less than one percent of our DNA varies from person to person, this can still leave important gaps and limit what we can learn from genomic analyses.
That’s why the concept of a pangenome has become increasingly popular. This refers to a collection of genomic sequences from many different people that have been merged to cover a much greater range of human genetic possibilities.

This AI Just Designed a More Precise CRISPR Gene Editor for Human Cells From Scratch
CRISPR was first discovered in bacteria as a defense mechanism, suggesting that nature hides a bounty of CRISPR components. For the past decade, scientists have screened different natural environments—for example, pond scum—to find other versions of the tool that could potentially increase its efficacy and precision. While successful, this strategy depends on what nature has to offer. Some benefits, such as a smaller size or greater longevity in the body, often come with trade-offs like lower activity or precision.
Rather than relying on evolution, can we fast-track better CRISPR tools with AI?
This week, Profluent, a startup based in California, outlined a strategy that uses AI to dream up a new universe of CRISPR gene editors. Based on large language models—the technology behind the popular ChatGPT—the AI designed several new gene-editing components.
Gene Editing Breakthrough: CRISPR Improves Vision in Clinical Trial
Jason Comander, MD, PhD, performs the procedure to deliver the CRISPR-based medicine as part of the BRILLIANCE trial in September 2020 at Mass Eye and Ear. Credit: Mass Eye and Ear.
All 14 trial participants, including 12 adults (ages 17 to 63) and two children (ages 10 and 14), were born with a form of Leber Congenital Amaurosis (LCA) caused by mutations in the centrosomal protein 290 (CEP290) gene. They underwent a single injection of a CRISPR/Cas9 genome editing medicine, EDIT-101 in one eye via a specialized surgical procedure. This trial, which included the first patient to ever receive a CRISPR-based investigational medicine directly inside the body, focused primarily on safety with a secondary analysis for efficacy.
No serious treatment or procedure-related adverse events were reported, nor were there any dose-limiting toxicities. For efficacy, the researchers looked at four measures: best-corrected visual acuity (BCVA); dark-adapted full-field stimulus testing (FST), visual function navigation (VNC, as measured by a maze participants completed), and vision-related quality of life.
The Stem Cell Podcast
In episode 267 of the Stem Cell Podcast, we chat with Dr. Shankar Srinivas, a Professor of Developmental Biology in the Department of Physiology Anatomy and Genetics based in the Institute for Developmental and Regenerative Medicine at the University of Oxford. He is also a Zeitlyn Fellow and Tutor in Medicine at Jesus College. Using mouse and human embryos as model systems, his group looks at the control of patterning and morphogenesis during the establishment of the anterior-posterior axis, gastrulation, and early cardiogenesis. He discusses how tissues respond to forces during early development, characterizing cardiac progenitors, and training internationally.
Roundup Papers:
2:26 https://bit.ly/3yeD3ms.
7:14 https://bit.ly/4dKJ7nd.
19:06 https://go.nature.com/3V2SNSo.
27:10 https://go.nature.com/4dnC43H
38:40 Guest Interview.
#Cardiogenesis #DevelopmentalBiology.
Listen on Apple Podcasts: https://apple.co/2T8BhPA
Listen on Stitcher: https://bit.ly/3hGwsGA
Listen on Spotify: https://spoti.fi/3xFdENP
Official Website: https://stemcellpodcast.com/