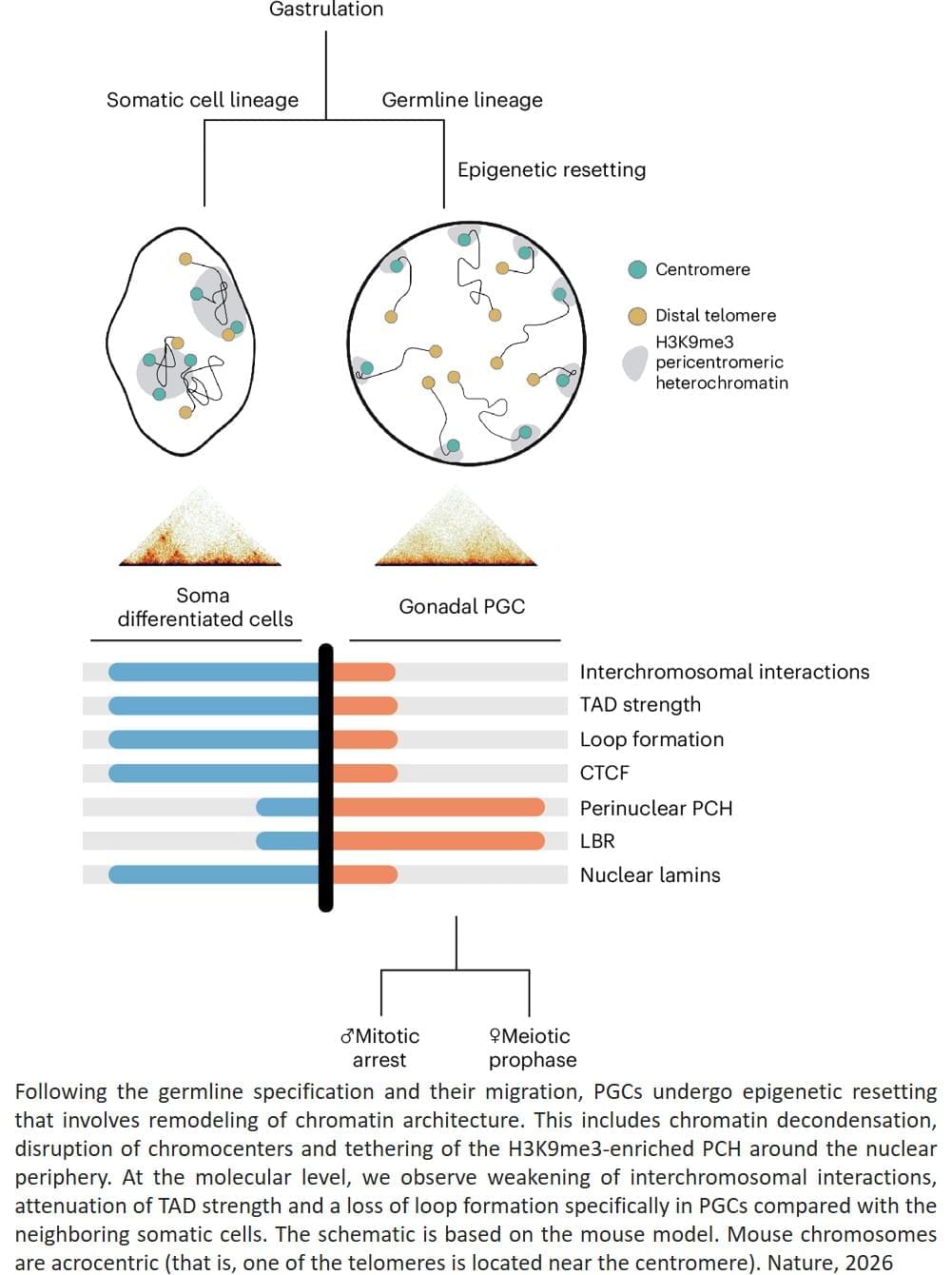
Using a technique called Hi-C analysis, which looks at how DNA is arranged in three dimensions inside the nucleus, the team found that at this transitional point the genome’s three-dimensional organisation becomes less structured and chromosomes become more separated inside the nucleus.
Creating sperm and eggs in the laboratory (in vitro) remains one of the greatest challenges in reproductive biology. To study this process, scientists use primordial germ cell–like cells (PGCLCs), which are lab-generated cells derived from embryonic stem cells that mimic the embryo’s earliest reproductive cells. However, these PCGLCs often fail to complete all the steps of meiosis, making it difficult to create functional sperm and eggs in petri dishes.
After studying the process in germ cells from the embryos, the team studied lab-generated mouse PCGLCs to see if the centromeres migrated to the periphery of the nucleus in vitro too, but they did not see the same phenomenon.
“The presence of this chromosome conformation in embryonic germ cells, but not lab-grown cells, suggests that this structural change could be required for meiosis to proceed properly, and could explain why meiosis is so difficult to recreate outside the body,” says the author, “but we need to do more work to fully characterise the process before we can say for sure.”
“Our study has uncovered a previously unknown and frankly very surprising restructuring of genome architecture that occurs in developing germ cells, which we believe is critical for a successful execution of meiosis,” says the senior author. ScienceMission sciencenewshighlights.
In our cells, our DNA carries chemical or ‘epigenetic’ marks that decide how genes will be used in different tissues. Yet in the group of specialised cells, known as ‘germ cells’, which will later form sperm and eggs, these inherited chemical instructions must be erased or reshuffled so development can begin again with a fresh blueprint in future generations.