How CDK2 inhibitors halt cancer cell division👇
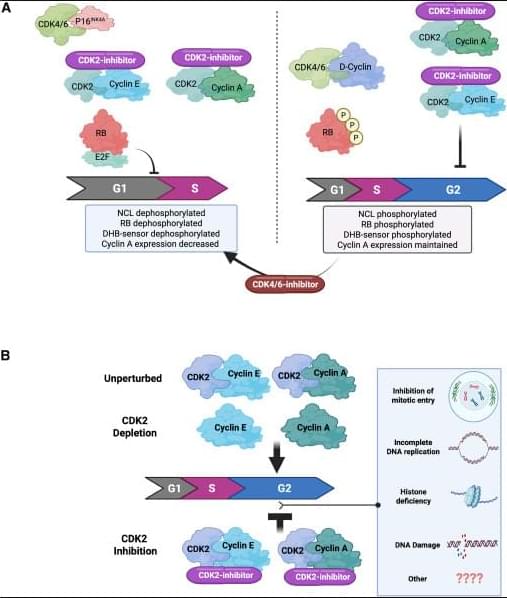
✅G1 arrest in p16INK4A-high, cyclin E–high tumors (A, left). In tumors with high p16INK4A and cyclin E, catalytic CDK2 inhibitors block phosphorylation of key CDK2 substrates, including RB and nucleolin, and reduce signals from CDK activity sensors. This suppresses E2F transcriptional activity, leading to reduced cyclin A expression and failure to enter S phase. The net result is a G1 cell-cycle arrest, driven by effective shutdown of the RB–E2F axis.
✅4N accumulation when p16INK4A is absent (A, right). In tumors lacking p16INK4A, CDK2 catalytic inhibition alone does not efficiently block RB phosphorylation or early G1 molecular events. Instead, cells continue through S phase and accumulate with 4N DNA content, indicating arrest later in the cycle (post-replication). In this context, adding a CDK4/6 inhibitor can mimic the p16INK4A state, restore RB dephosphorylation, repress E2F, and shift cells toward a G1 arrest, highlighting the importance of dual CDK control of RB.
✅Catalytic inhibition vs genetic depletion of CDK2 (B). Genetic loss of CDK2 is often tolerated because cells can compensate using cyclin A–CDK1 to complete G2/M. In contrast, catalytic CDK2 inhibitors trap CDK2 in inactive complexes with cyclins, which may interfere with normal handoff to CDK1 and other cell-cycle processes. This leads to accumulation of cells with 4N DNA content, reflecting a block after DNA replication.
✅Why the outcomes differ. These findings suggest that CDK2 has roles beyond simple kinase activity—its inactive, cyclin-bound state under catalytic inhibition may disrupt network dynamics differently than complete protein loss. The precise mechanisms of the 4N arrest are still being investigated and may involve defects in S/G2 transitions, replication stress responses, or mitotic entry control.
✅Therapeutic implication. Tumor response to CDK2 inhibitors depends strongly on p16INK4A status, cyclin E levels, and RB pathway integrity. This supports combination strategies (CDK2 + CDK4/6 inhibition) in selected cancers and emphasizes the need for biomarker-guided patient stratification.