Insect-killing fungi are used to control insect pests worldwide. Scientists can genetically engineer them to be even more effective and suited for our needs.


But CRISPR editing — at least as a therapeutic technique in people — has turned out to be more difficult than initially thought. Researchers have documented ways that Cas9, one of the enzymes used in CRISPR gene editing, could trigger immune responses, or cause accidental changes to the genome that would be permanent. RNA editing, by contrast, could allow clinicians to make temporary fixes that eliminate mutations in proteins, halt their production or change the way that they work in specific organs and tissues. Because cells quickly degrade unused RNAs, any errors introduced by a therapy would be washed out, rather than staying with a person forever.
Making changes to the molecular messengers that create proteins might offer flexible therapies for cancer, pain or high cholesterol, in addition to genetic disorders.

Bloom Science and Duke University have entered into an exclusive licensing agreement that provides the biopharmaceutical company access to the intellectual property and technology related to unique strain isolates and genetic variants of Akkermansia genus bacteria.
This type of bacteria has been demonstrated to slow disease progression and prolong survival in animal models of amyotrophic lateral sclerosis (ALS).
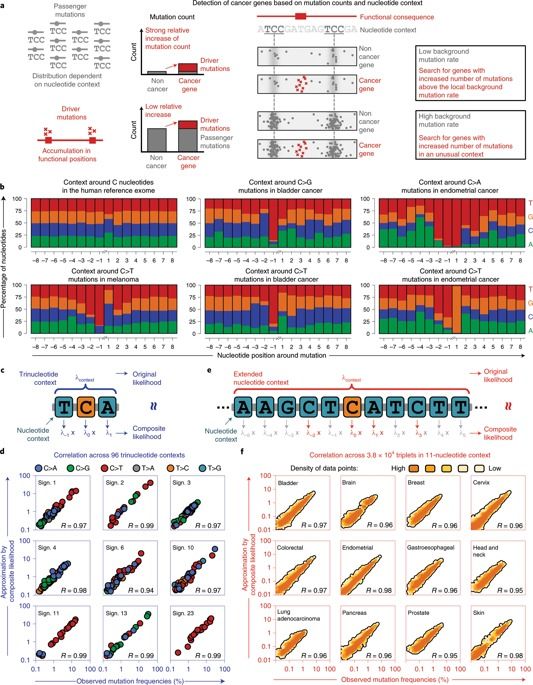
Cancer genomes contain large numbers of somatic mutations but few of these mutations drive tumor development. Current approaches either identify driver genes on the basis of mutational recurrence or approximate the functional consequences of nonsynonymous mutations by using bioinformatic scores. Passenger mutations are enriched in characteristic nucleotide contexts, whereas driver mutations occur in functional positions, which are not necessarily surrounded by a particular nucleotide context. We observed that mutations in contexts that deviate from the characteristic contexts around passenger mutations provide a signal in favor of driver genes. We therefore developed a method that combines this feature with the signals traditionally used for driver-gene identification. We applied our method to whole-exome sequencing data from 11,873 tumor–normal pairs and identified 460 driver genes that clustered into 21 cancer-related pathways. Our study provides a resource of driver genes across 28 tumor types with additional driver genes identified according to mutations in unusual nucleotide contexts.
Researchers from SENS Research Foundation, including Matthew O’Connor and Amutha Boominathan, have published a new study showing how codons play an important role in getting copies of mitochondrial genes placed in the cellular nucleus to express themselves correctly [1].
A possible solution to mitochondrial diseases
Mitochondrial disease is not a single disease; in fact, it is a group of rare and related conditions that are thought to affect perhaps 1 in 5000 people. These are caused due to mutations in the genes involved in the process of aerobic respiration, one of the main functions of our mitochondria.
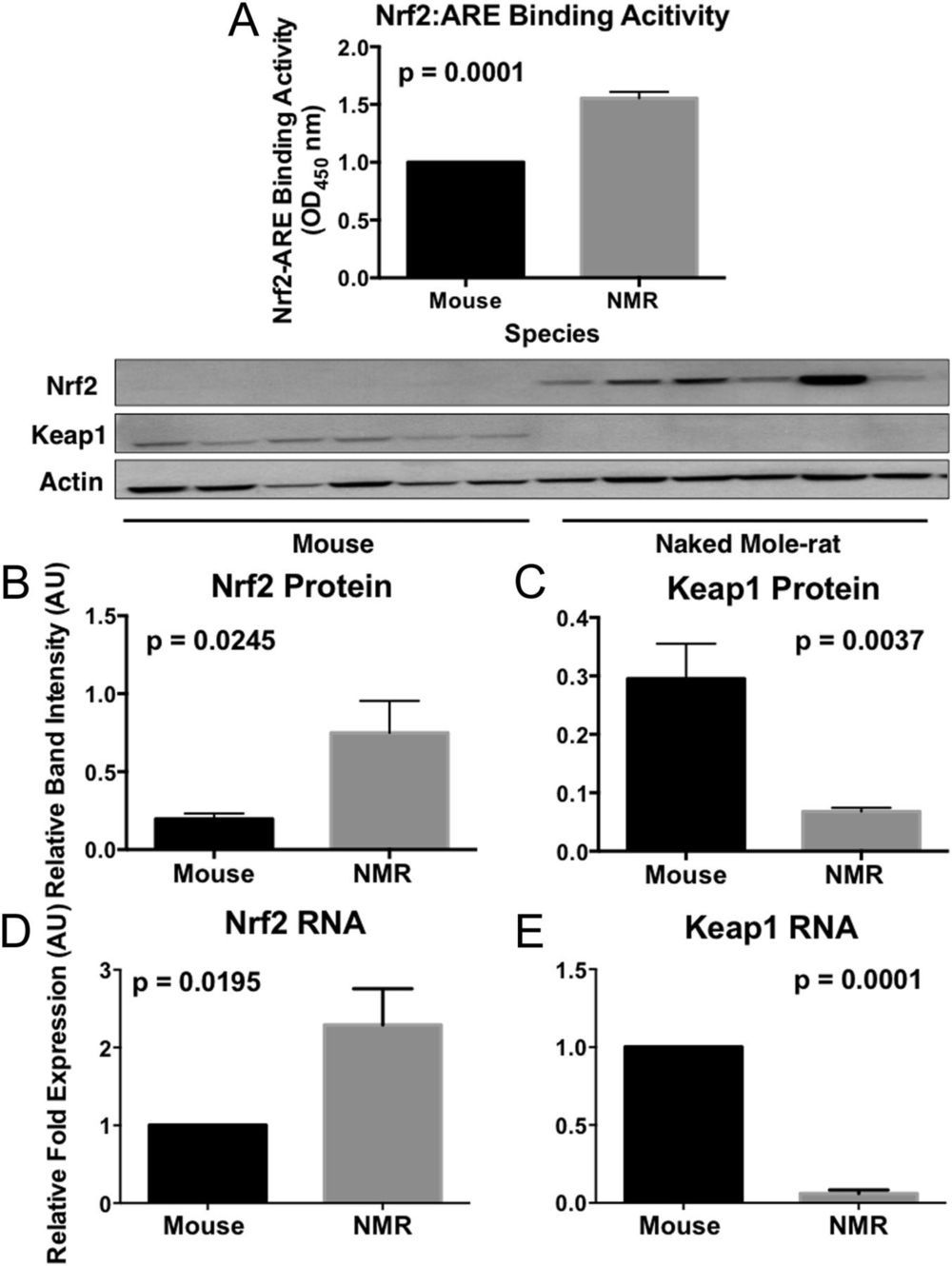
Both genetically altered and naturally long-lived mammals are more resistant to toxic compounds that may cause cancer and age-associated diseases than their shorter-lived counterparts. The mechanisms by which this stress resistance occurs remain elusive. We found that longer-lived rodent species had markedly higher levels of signaling activity of the multifunctional regulator nuclear factor erythroid 2-related factor (Nrf2) and that this increase in cytoprotective signaling appeared to be due to species differences in Kelch-like ECH-Associated Protein 1 (Keap1) and β-transducin repeat-containing protein (βTrCP) regulation of Nrf2 activity. Both of these negative regulators of Nrf2-signaling activity are significantly lower in longer-lived species. By targeting the proteins that regulate Nrf2 rather than Nrf2 itself, we may be able to identify new therapies that impact aging and age-associated diseases such as cancer.
The preternaturally long-lived naked mole-rat, like other long-lived species and experimental models of extended longevity, is resistant to both endogenous (e.g., reactive oxygen species) and environmental stressors and also resists age-related diseases such as cancer, cardiovascular disease, and neurodegeneration. The mechanisms behind the universal resilience of longer-lived organisms to stress, however, remain elusive. We hypothesize that this resilience is linked to the activity of a highly conserved transcription factor, nuclear factor erythroid 2-related factor (Nrf2). Nrf2 regulates the transcription of several hundred cytoprotective molecules, including antioxidants, detoxicants, and molecular chaperones (heat shock proteins). Nrf2 itself is tightly regulated by mechanisms that either promote its activity or increase its degradation.


The drug, known as DSP-1181, was created by using algorithms to sift through potential compounds, checking them against a huge database of parameters, including a patient’s genetic factors. Speaking to the BBC, Exscientia chief executive Professor Andrew Hopkins described the trials as a “key milestone in drug discovery” and noted that there are “billions” of decisions needed to find the right molecules for a drug, making their eventual creation a “huge decision.” With AI, however, “the beauty of the algorithm is that they are agnostic, so can be applied to any disease.”
We’ve already seen multiple examples of AI being used to diagnose illness and analyze patient data, so using it to engineer drug treatment is an obvious progression of its place in medicine. But the AI-created drugs do pose some pertinent questions. Will patients be comfortable taking medication designed by a machine? How will these drugs differ from those developed by humans alone? Who will make the rules for the use of AI in drug research? Hopkins and his team hope that these and myriad other questions will be explored in the trials, which will begin in March.

Circa 2013
Tigers and their close relatives (Panthera) are some of the world’s most endangered species. Here we report the de novo assembly of an Amur tiger whole-genome sequence as well as the genomic sequences of a white Bengal tiger, African lion, white African lion and snow leopard. Through comparative genetic analyses of these genomes, we find genetic signatures that may reflect molecular adaptations consistent with the big cats’ hypercarnivorous diet and muscle strength. We report a snow leopard-specific genetic determinant in EGLN1 (Met39Lys39), which is likely to be associated with adaptation to high altitude. We also detect a TYR 260GA mutation likely responsible for the white lion coat colour. Tiger and cat genomes show similar repeat composition and an appreciably conserved synteny. Genomic data from the five big cats provide an invaluable resource for resolving easily identifiable phenotypes evident in very close, but distinct, species.

“It regenerates almost anything after almost any injury that doesn’t kill it,” said Parker Flowers, postdoctoral associate in the lab of Craig Crews, the John C. Malone Professor of Molecular, Cellular, and Developmental Biology and professor of chemistry and pharmacology.
If scientists can find the genetic basis for the axolotl’s ability to regenerate, they might be able to find ways to restore damaged tissue in humans. But they have been thwarted in the attempt by another peculiarity of the axolotl — it has the largest genome of any animal yet sequenced, 10 times larger than that of humans.
Now Flowers and colleagues have found an ingenious way to circumvent the animal’s complex genome to identify at least two genes involved in regeneration, they report Jan. 28 in the journal eLife.