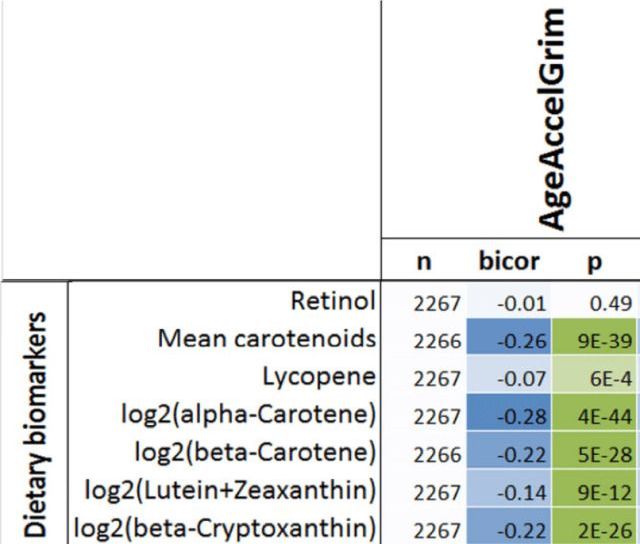
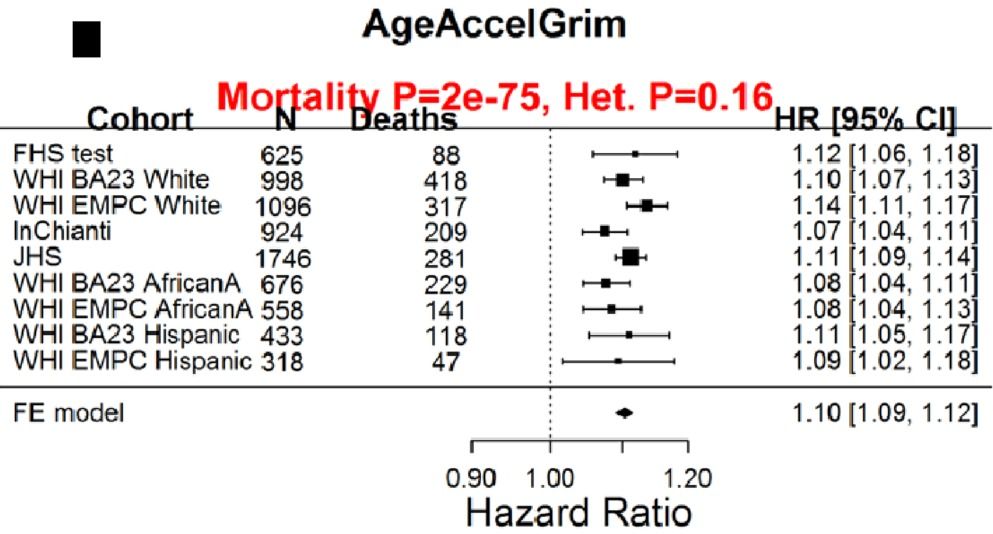
The quest to live longer and healthier is not new. But the concept of reversing aging has recently stunned both the scienftific community and the public in general. Scientists have been able to reverse aging by 2.5 years to some participants in a groundbreaking experiment in the field of age reversal.
World leading scientists in the field of aging like David Sinclair think that aging is the ultimate disease that needs a cure. If scientsits were able to shed 2.5 years to the participants genomic age, the question raises itself, are we going to see an age reversal of a decade or more in the coming years?
#reverseaging #science #sciencetime
SUBSCRIBE to our channel “Science Time” and ring the bell to never miss out videos like this: https://www.youtube.com/sciencetime24
Source: Fahy, G. M. et al. Aging Cell https://doi.org/10.1111/acel.13028 (2019)
Similar videos: