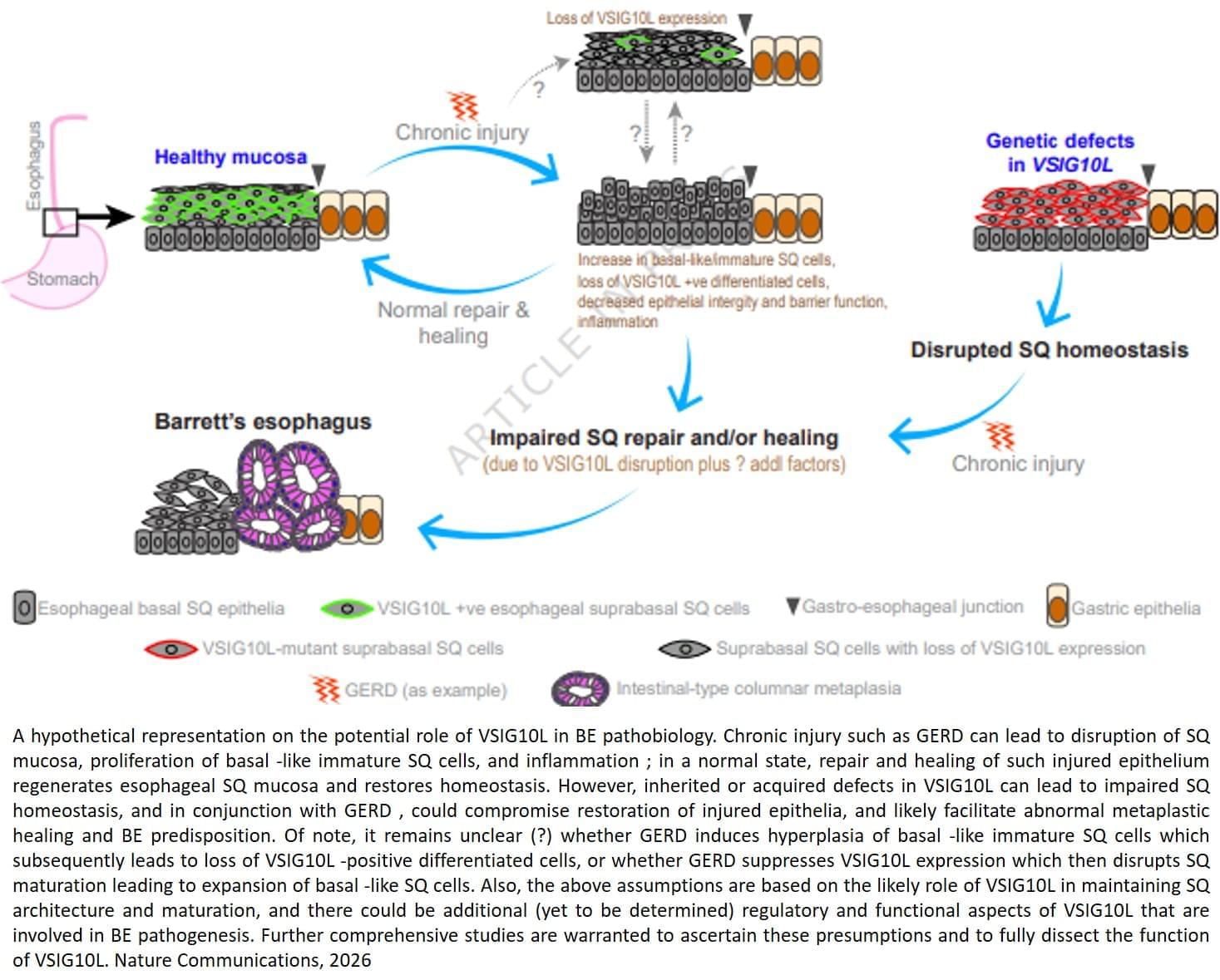
But the molecular factors responsible for the onset of Barrett’s esophagus remain poorly understood.
The findings, published in Nature Communications, combined family studies, laboratory experiments and genetically engineered mouse models to identify and understand how genetic defects contribute to disease development.
The team sequenced and analyzed genetic material of 684 people from 302 families where multiple members developed Barrett’s esophagus or esophageal cancer. They discovered that a subset of affected family members carry inherited mutations in a gene called VSIG10L.
“We found that this gene acts like a quality control system for the esophageal lining,” said the lead researcher. “When it’s defective, the cells do not mature properly and the protective barrier in the esophageal lining becomes weak, allowing stomach bile acid to cause tissue changes that enhances the risk of developing Barrett’s esophagus.”
When researchers genetically engineered mice with human-equivalent VSIG10L mutations, they found that the esophageal lining became disrupted structurally and molecularly, according to the author. The study found that when the mice were exposed to bile acid, they developed Barrett’s-like disease over time, effectively replicating the disease’s progression in humans.
These genetically engineered mice also represent the first animal model for Barrett’s esophagus based directly on human genetic predisposition to the disease, the author said.
With VSIG10L shown to be a key gene in maintaining esophageal health, family members can now be screened for genetic variants to identify those at a high-risk of developing Barrett’s esophagus or esophageal cancer. ScienceMission sciencenewshighlights.