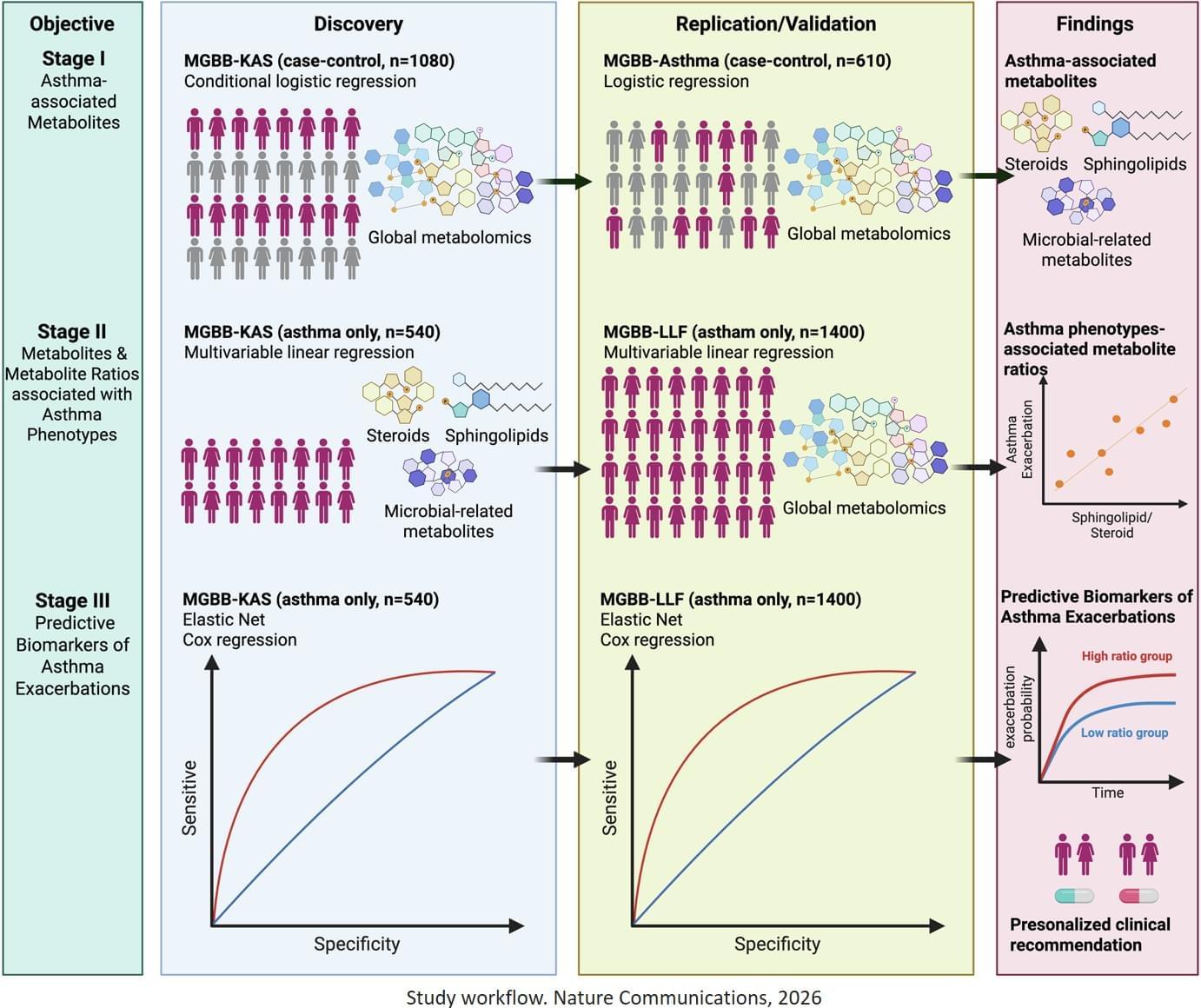
“One of the biggest challenges in treating asthma is that we currently have no effective way to tell which patient is going to have a severe attack in the near future,” says the senior author. “Our findings solve a critical unmet need. By measuring the balance between specific sphingolipids and steroids in the blood, we can identify high-risk patients with 90 per cent accuracy, allowing clinicians to intervene before an attack occurs.”
The team discovered that while individual metabolite levels provided some insight, the ratio between sphingolipids and steroids was the most powerful predictor of future health. ScienceMission sciencenewshighlights.
Researchers have identified a new method to predict asthma exacerbations with a high degree of accuracy. The study is published in Nature Communications.
Asthma is one of the world’s most common chronic diseases, affecting over 500 million people. Asthma exacerbations – commonly known as asthma attacks – are a major cause of disease morbidity and healthcare costs. Despite the prevalence of asthma, clinicians currently lack reliable biomarkers to identify which patients are at high risk for future attacks. Current methods often fail to distinguish between stable patients and those prone to severe exacerbations.
The study analysed data from three large asthma cohorts totalling over 2,500 participants, backed by decades of electronic medical records. Researchers used a high throughput approach called metabolomics to measures small molecules in the blood of individuals with asthma. They identified an important relationship between two classes of metabolites, sphingolipids and steroids, and asthma control. Specifically, they identified that sphingolipid to steroid ratios could predict exacerbation risk over a 5-year period. In some cases, the model could differentiate the time-to-first exacerbation between high-and low-risk groups by nearly a full year.