Age-related chromatin remodeling includes shared and specific signatures across cell types, sex, and organs


Cellular reprogramming is one of the technologies most associated with longevity. The field was created in 2006, when Shinya Yamanaka showed that a cocktail of four transcription factors, commonly known as OSKM, can cause de-differentiation and massive rejuvenation of a cell, creating an iPSC (induced pluripotent stem cell). About a decade later, partial reprogramming was demonstrated in vivo, where a more subtle application of the factors led to rejuvenation without compromising the cell’s identity.
Today, this field is maturing quickly, with its first clinical trials just around the corner. Academic teams and companies are working on dozens of directions and applications. We asked four experts, all involved in reprogramming-related biotech companies, to talk about their companies’ approaches and the opportunities and bottlenecks that the field faces and to offer predictions for the near and not-so-near future.
What I find most compelling about cellular reprogramming is that it revealed aging to be, at least in part, an actively maintained biological state rather than irreversible accumulation of damage. The discovery that somatic cells retain a latent capacity to reset their epigenetic and functional identity fundamentally changed how we think about cellular plasticity, identity, and time.
Groundbreaking research reveals senolytics combined with stem cells can double lifespan. This isn’t a small improvement; it’s a revolutionary leap in regenerative medicine, supporting a new paradigm. #Longevity #RegenerativeMedicine #StemCells #Senolytics #HealthTech @immortabio
Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
Blood testing with LifeExtension.com: https://www.anrdoezrs.net/click-101614996-15750394
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
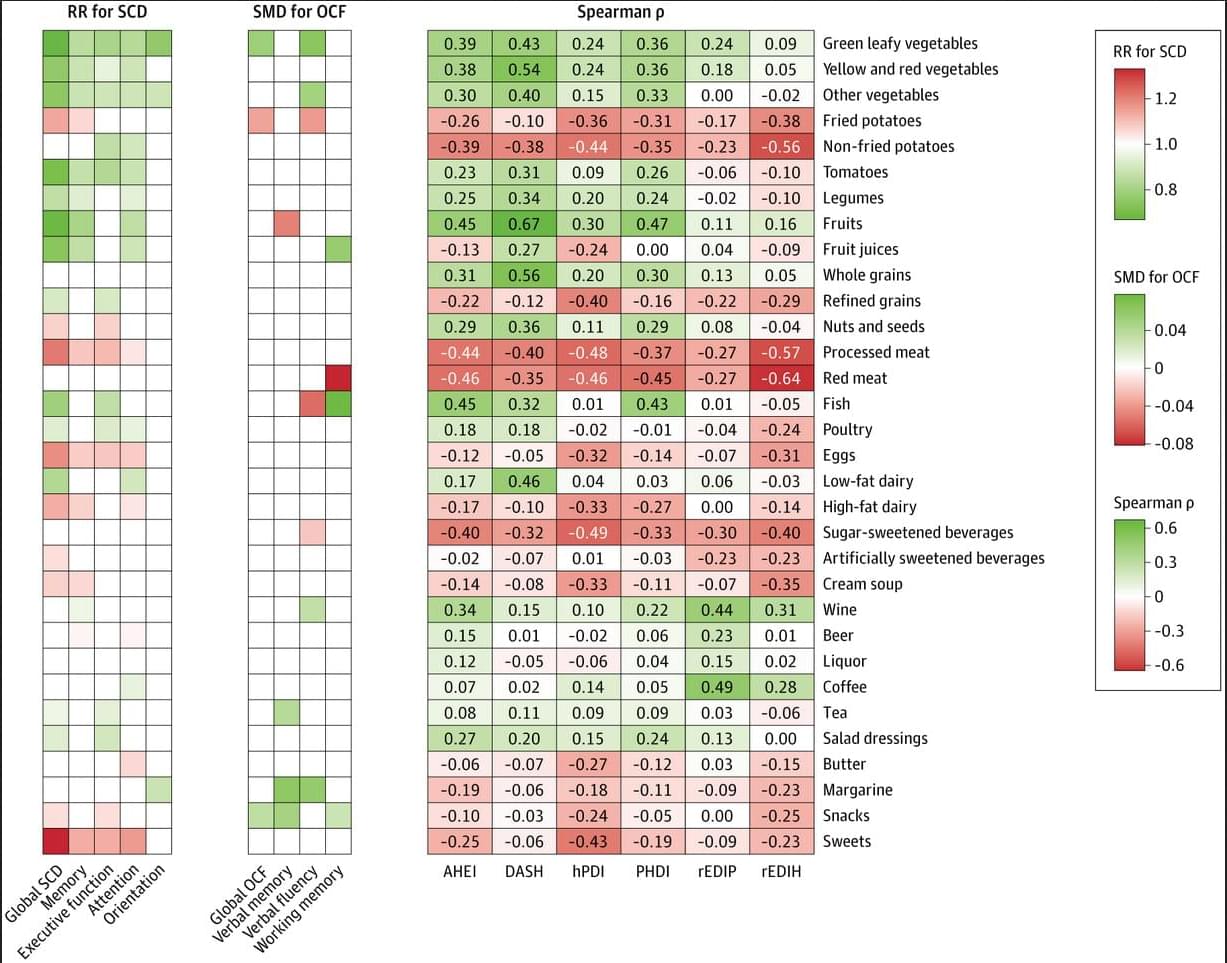
Greater adherence to the DASH diet, plant-based dietary patterns, or diets with lower hyperinsulinemia and inflammation was associated with lower risk of subjective cognitive decline and better cognitive function in adults.
Question Are healthy dietary patterns associated with lower risk of subjective cognitive decline and better objectively measured cognitive function?
Findings In this cohort study performing a systematic evaluation of 6 dietary patterns among 159 347 participants, greater adherence to a healthy diet, exemplified by the Dietary Approaches to Stop Hypertension (DASH) diet, was associated with lower risk of subjective cognitive decline and better objectively measured cognitive function. The associations were most pronounced when the diet was followed during midadulthood (ages 45–54 years).
Meaning Results suggest that a healthy diet, such as the DASH diet, was associated with early indicators of cognitive aging, which underscores the importance of a healthy diet for maintaining long-term cognitive health.
Immorta Bio just doubled the lifespan of mice using a first-in-class senolytic immunotherapy called SenoVax combined with personalized stem cells from their StemCellRevivify platform. In this deep dive, I break down exactly how it works, why it matters, and what it means for the future of human aging.
SenoVax is a vaccine that trains your immune system to hunt down and destroy senescent cells — the \.

Amin et al. present telomere-to-telomere assemblies of nine Borrelia burgdorferi strains from Canada, uncovering the diversity at chromosome ends and plasmid profiles. They identify an lp28-1a plasmid subtype and detect plasmid-chromosome recombination, offering insights into genomic plasticity and potential mechanisms of adaptation in Borrelia.
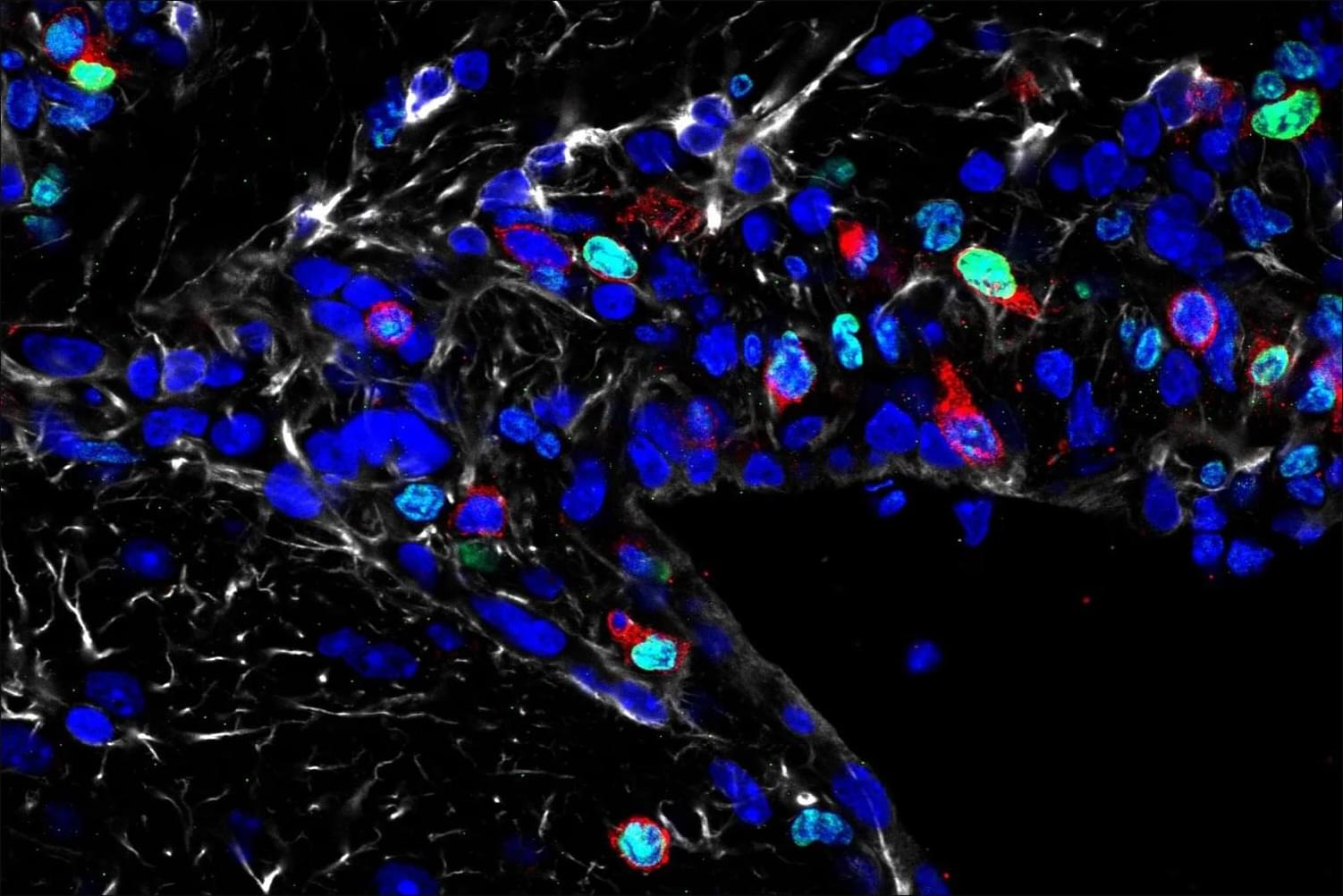
Researchers at Stanford University engineered a modified version of the immune protein interleukin-10 (IL-10) that retains only its anti-inflammatory properties while eliminating its pro-inflammatory ones. When injected into aged mice, this modified protein stimulated the growth of new neurons and improved performance on memory and learning tasks, such as maze navigation and object recognition. The study, published in Immunity, suggests that age-related cognitive decline is linked to the accumulation of exhausted T-lymphocytes in the brain, chronic inflammation, and impaired microglial function — all of which reduce neurogenesis. The findings indicate that selectively modulating immune signaling could open new avenues for treating neurodegenerative diseases. The team plans to further investigate the protein’s mechanisms and explore ways to target specific cell types more precisely to minimize potential side effects.
A modified immune protein developed by Stanford researchers points to a novel strategy for combating age-related cognitive decline.

Fifteen years ago, I wrote something that annoyed many techno-optimists.
Ten years ago, I filmed it as a podcast.
Today it feels less controversial — and more urgent.
Technology is NOT Enough.
We have the science to feed everyone. We have the tech to provide clean water. We understand climate change. We know how to reduce suffering.
And yet we don’t act.
