Practice teams are urged to share details with clients about this canine longevity study, whose long-term goal is to help pets and people live longer, healthier lives.



Our friends at Foresight Institute and 100 Plus Capital have started regular longevity salons to coordinate the growing longevity enthusiasm and onboard new investors into the space. You are invited to kick off this series with aperitifs and hors d’oeuvre to discuss the current innovations that have been fueling the recent optimism in the field:
Longevity Investment Take-Off: What’s Different This Time, What’s Missing?
As a friend of Lifespan.io, you are welcome to use the code lifespan.io for a 50% discount on the ticket price.

Congratulations to Osinakachi Gabriel for his launch of the first publication the TAFFD’s “Magazine of the Future” — Also thanks for the Bioquark (page 37) and Regenerage (page 72) profiles — https://issuu.com/taffds/docs/taffd_s_magazine_2019 #Futurism #Longevity #Transhumanism #Biotechnology #Health #Wellness #Regeneration #LifeExtension #Aging #Immortality #IraPastor #Bioquark #Regenerage #Ideaxme #Singularity #Consciousness #AI #JasonSilva #ArtificiaIIntelligence #SENS
In this first issue by Trandisciplinary Agora For Future Discussions, we approach reality from a transdisciplinary perspective in order to find unity and greater understanding of the world as we enter a new paradigm in technological advancements that will lead us to transcending our own biology while enhancing our mental and physical limitations. We explore all topics that relate to transhumanism, cybernetic singularity, energy, consciousness, international policy, electromagnetic forces, language, AI, digitalization, ethics, philosophy, biotechnology, futurism and more.
A Yale University experiment, led by neuroscientist Nenad Sestanwhich reawakened the brains of slaughtered pigs has raised speculation that human trials could be next, renewing ethical concerns over the pursuit of immortality. In the experiments, the pigs did not regain consciousness but Sestan acknowledged that restoring awareness is a possibility and that the technique could work on humans, keeping the brain alive indefinitely.
Nottingham Trent University ethics researcher Benjamin Curtis says ending up as a disembodied brain might just be a “living hell.” Writing in The Conversation he suggested that living without any actual contact with reality could be a fate worse than death. “Some have argued that even with a fully functional body, immortality would be tedious. With absolutely no contact with external reality, it might just be a living hell,” Curtis wrote.
Curtis explained that the brain is highly integrated with the rest of the body in both humans and animals. It is constantly receiving and sending signals from and to it. “We have no idea what experiences would occur within a disembodied brain. But those experiences may well be deeply disturbing,” he said.
In a surprising study, Oregon State University researchers found that no matter how much stress they placed on mice from either a high-fat diet or strenuous exercise, the animals’ mitochondria were able to adapt and continue their normal processes.
The findings could have major implications for the study of diseases like diabetes, Parkinson’s and Alzheimer’s, all of which are associated with an impairment in the breaking-down and clearance of damaged mitochondria.
Mitochondria are the structures that house cellular respiration, the process used to turn nutrients into energy. Dysfunction in mitochondria may lead to lower energy production, greater inflammation and tissue damage. Yet as central as mitochondria are to living organisms, scientists still don’t know exactly what keeps them healthy—or makes them unhealthy.
You may have noticed that the LEAF website is no longer there! Don’t panic, we will explain everything.
We have come a long way
Back in late 2016, we launched the LEAF website as a companion site to Lifespan.io, our research fundraising platform. Since those first steps, the site has become increasingly popular and has enjoyed rapid growth during this time.
Identifying biological correlates of late life cognitive function is important if we are to ascertain biomarkers for, and develop treatments to help reduce, age-related cognitive decline. Here, we investigated the associations between plasma levels of 90 neurology-related proteins (Olink® Proteomics) and general fluid cognitive ability in the Lothian Birth Cohort 1936 (LBC1936, N = 798), Lothian Birth Cohort 1921 (LBC1921, N = 165), and the INTERVAL BioResource (N = 4451). In the LBC1936, 22 of the proteins were significantly associated with general fluid cognitive ability (β between −0.11 and −0.17). MRI-assessed total brain volume partially mediated the association between 10 of these proteins and general fluid cognitive ability. In an age-matched subsample of INTERVAL, effect sizes for the 22 proteins, although smaller, were all in the same direction as in LBC1936. Plasma levels of a number of neurology-related proteins are associated with general fluid cognitive ability in later life, mediated by brain volume in some cases.
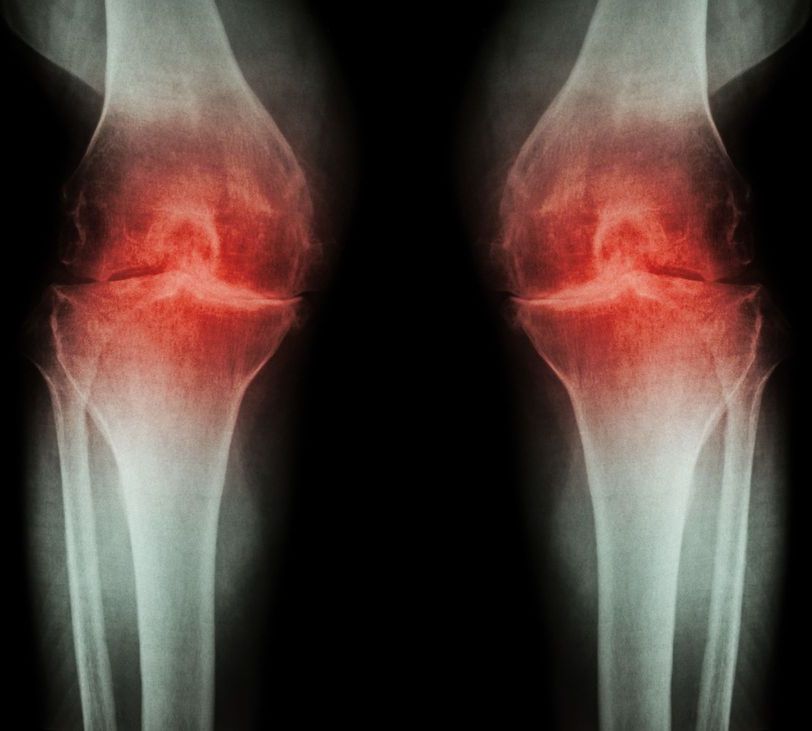
Osteoarthritis is one of the most common ailments of an aging population, but there aren’t many treatment options besides taking pain killers or getting a full joint replacement. Now, researchers at the Salk Institute have found that a combination of two experimental drugs appears to reverse the symptoms of the disorder, with successful tests conducted in rats and in human cells in the lab.
As we age, our bodies gradually lose the ability to repair damage as fast as it needs to. That means that tissues subjected to high-intensity long-term use, such as cartilage in joints, are especially prone to wearing out. That manifests itself in the common condition of osteoarthritis, resulting in pain when that joint moves.
In the past, two molecules have been identified as having decent potential for treating the disease – alpha-KLOTHO (αKLOTHO) and TGF beta receptor 2 (TGFβR2). Previous tests only showed moderate success, so the researchers on the new study decided to see if they fared any better when used together.