The ILA welcomes its new federated member – the Italian Longevity League, Italy!
https://ec.europa.eu/eip/ageing/sites/eipaha/themes/eiponaha2/logo.png]

The ILA welcomes its new federated member – the Italian Longevity League, Italy!
https://ec.europa.eu/eip/ageing/sites/eipaha/themes/eiponaha2/logo.png]

Epigenetic reprogramming. “We are very radical life extensionists, neither healthspan increase no morbidity compression are enough, we would like to add decades if not centuries to the human lifespan.”
Yuri Deigin Founder of Youthereum, speaking at Master Investor’s Investing in the Age of Longevity 2019 event.
Master Investor is an investment media and events company that delivers independent, financial commentary and analysis to UK private investors and traders through events, magazines, news, blogs, podcasts and daily/weekly newsletters.
Read our latest magazine at www.masterinvestor.co.uk/magazine
Book a free ticket (use code MIYT) to attend the annual Master Investor Show (28th March 2020): https://masterinvestorshow-2020.reg.buzz

Once you hit a certain age you start to see subtle changes and many begin to search for options to hold onto the appearance of youthfulness for as long as they can in the form of lotions, potions, creams, supplements, serums, diets, and concoctions among others.
Soon there may be a new addition to the anti-aging lineup, that being rapamycin which is an FDA approved drug that is normally used to prevent organ rejection after transplant, the drug may also be helpful in slowing in aging skin according to a recent study published in Geroscience.
Studies have used rapamycin to effectively slow aging in worms, flies, and mice but this study from Drexel University College of Medicine is the first to show an effect on aging in human tissues, specifically the skin; findings showed signs of aging to be reduced including decreases in wrinkles, reduced sagging, and more even skin tone when delivered to humans topically.

5 European startups extending our lifespans ~ via EU-Startups #perpetuallife
How can we live longer? 5 European startups extending our lifespans
How can we live longer? How do we live healthily to extend our lifespans? How do we feel younger than our age? These are among the many questions scientists have been trying to answer about aging.

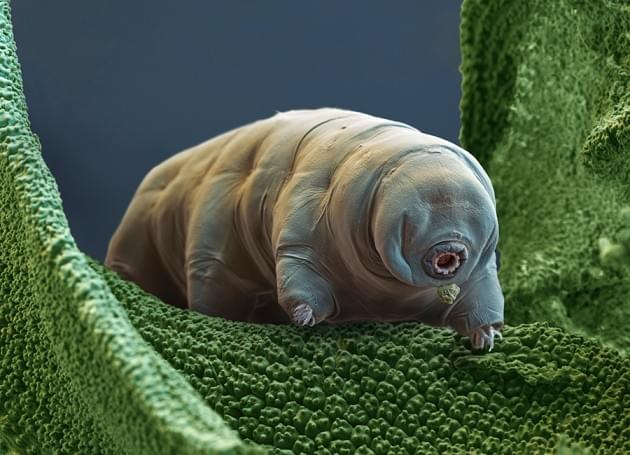
The researchers wanted to know how tardigrades protected themselves against such harsh conditions. So Kunieda and his colleagues began by sequencing the genome of Ramazzottius varieornatus, a species that is particularly stress tolerant. It’s easier to study processes within the tardigrade’s cells when the animal’s genome is inserted into mammalian cells, says Kunieda. So researchers manipulated cultures of human cells to produce pieces of the water bear’s inner machinery to determine which parts were actually giving the animals their resistance.
Eventually, Kunieda and his colleagues discovered that a protein known as Dsup prevented the animal’s DNA from breaking under the stress of radiation and desiccation. And they also found that the tardigrade-tinged human cells were able to suppress X-ray induced damage by about 40%.
“Protection and repair of DNA is a fundamental component of all cells and a central aspect in many human diseases, including cancer and ageing,” says Ingemar Jönsson, an evolutionary ecologist who studies tardigrades at Kristianstad University in Sweden.

The Race To Find A Cure For Aging Learn about three pioneers working to turn back the clock ~ via Medium #perpetuallife
https://medium.com/discourse/the-race-to-find-a-cure-for-aging-98676b0318dc
We want to look & feel young again, and every year we spend hundreds of billions of dollars on beauty serums, cosmetic surgery, and exotic supplements in the hopes of appearing more vibrant, healthy, and desirable.
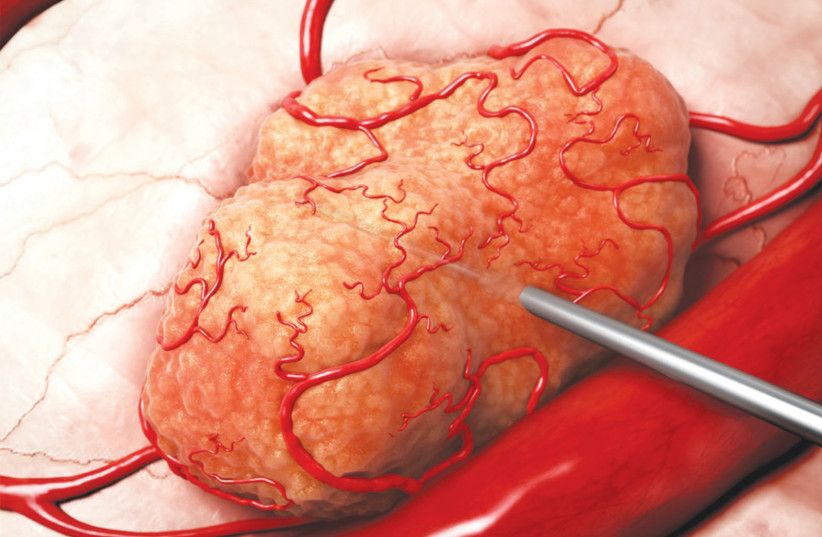
The second thing to mention is that in many instances, we actually have cracked it, at least medically. There are endless cutting-edge treatments being deployed as we speak, and endless additional cutting-edge research projects being conducted. There are new breakthroughs all the time, much of which are awaiting FDA approval, but one thing many of these treatments and cures have in common is that they are developed in a tiny country smaller than New Jersey that happens to be located in perhaps the worst neighborhood on Earth. When it comes to cancer research, Israel leads the way.
Finally, the last time I wrote about cutting-edge cancer treatments, I was contacted by a close friend who is a leading oncologist. He pointed out that a disclaimer is very much necessary when I write these articles. The last thing oncologists need is for their patients to think there is some magical solution here and show up to their appointments with a cut-out of an article. In fact, the last thing a cancer patient needs is false hope.
So the disclaimer is that this company is in the clinical trial phase, and if all goes according to plan, tumors might be much more manageable and treatable than they are today, but a lot has to happen before we get there.