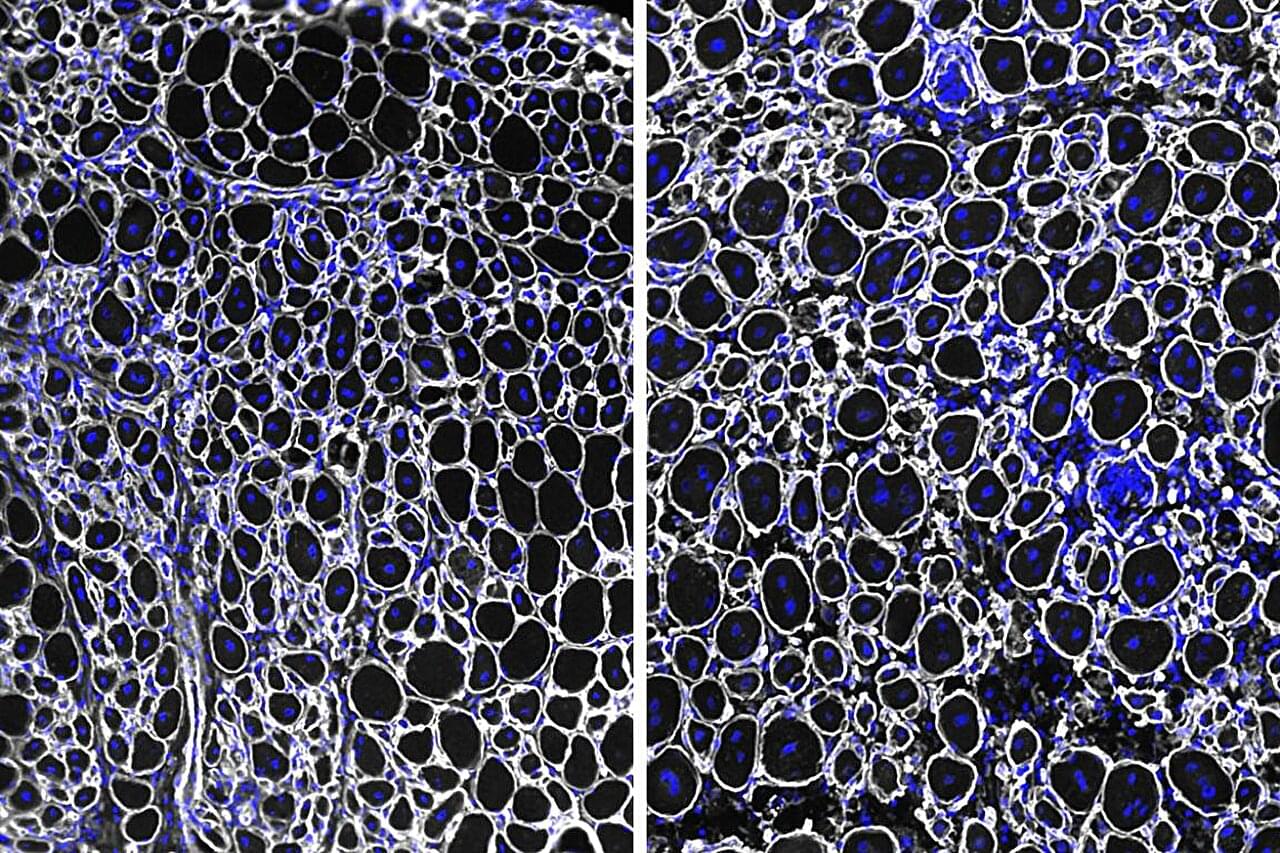
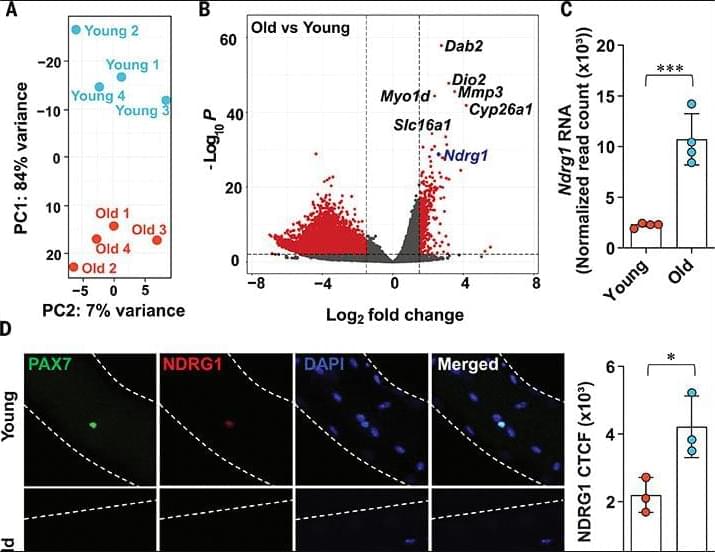
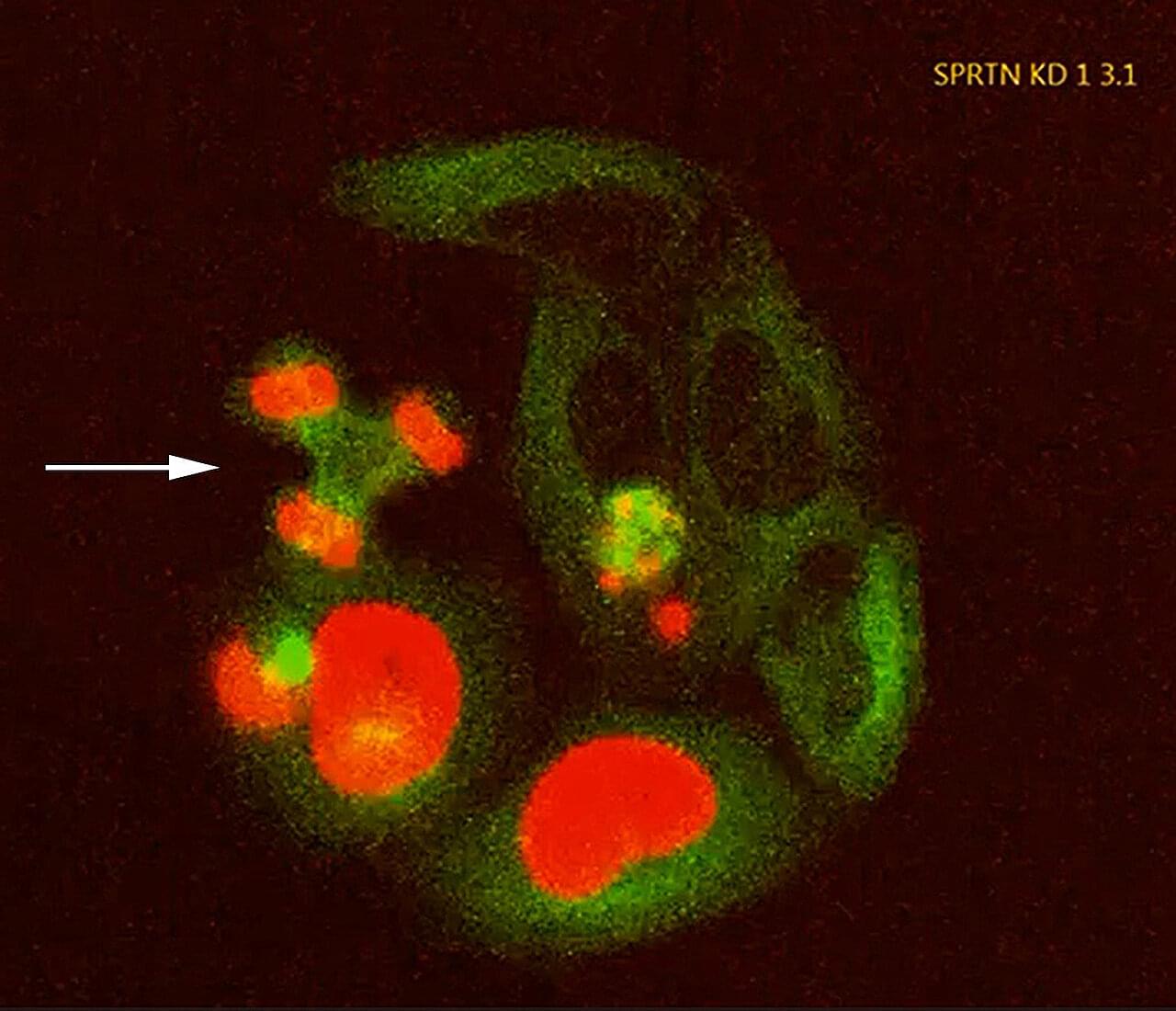
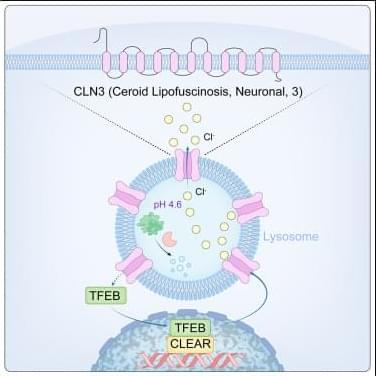
Aging muscles heal more slowly after injury—a frustrating reality familiar to many older adults. A UCLA study conducted in mice reveals an unexpected cause: Stem cells in aged muscle accumulate higher levels of a protein that slows their ability to activate and repair tissue, but helps the cells survive longer in the harsh environment of aging tissue.
The findings, published today in the journal Science, suggest that some molecular changes associated with getting older may actually be protective adaptations rather than purely detrimental effects.
“This has led us to a new way of thinking about aging,” said Dr. Thomas Rando, senior author of the new study and director of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA.