http://innergameofaging.com/iga36



The SRF Summer Scholars Program offers undergraduate students the opportunity to conduct biomedical research to combat diseases of aging, such as cancer, atherosclerosis, and Parkinson’s Disease. Under the guidance of a scientific mentor, each Summer Scholar is responsible for his or her own research project in such areas as genetic engineering and stem cell research. The Summer Scholars Program emphasizes development of both laboratory and communication skills to develop well-rounded future scientists, healthcare professionals, and policy makers.
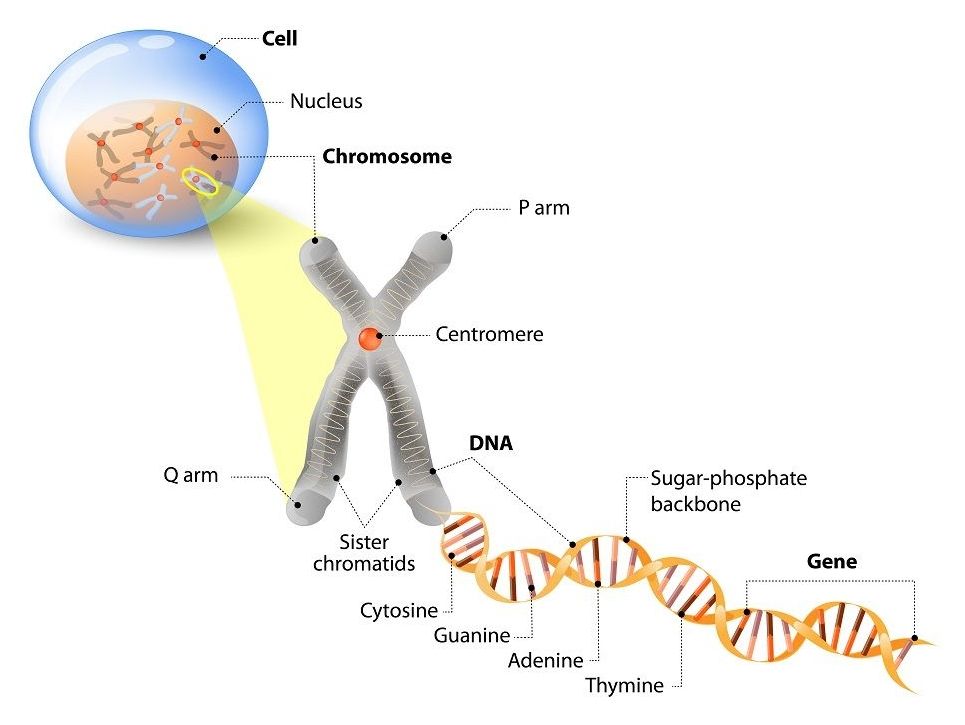
This is the third part of our ongoing series of articles that discuss the Hallmarks of Aging. Published in 2013, the paper divides aging into distinct categories (“hallmarks”) of damage to explain how the aging process works and how it causes age-related diseases[1].
Today, we will be looking at one of the primary hallmarks, telomere attrition.

A number of studies in different countries show that when people are asked “how long would you like to live?”, they respond with a figure equal to or slightly higher than the current life expectancy in a given country[1–4]. So, why does the public often lack enthusiasm for longevity?
These studies have shown that, generally, the public is uninterested in living longer than normal because they believe that these extra years will be spent suffering from the illnesses of old age. This is why the public often reacts to words like ‘longevity’ this way; to them, ten extra years likely means a decade spent in a wheelchair or some other decrepit state robbed of independence and health.

According to a French physiologist, humans have reached the peak of our height, lifespan and physical fitness.
I suspect that from our vantage point (a narrow snapshot of human evolution), we lack sufficient data to arrive this sweeping conclusion. Nevertheless, mainstream media is taking this research seriously.
http://www.newsweek.com/humans-reached-peak-height-lifespan-fitness-741816

Ray Kurzweil discusses how biotechnology is rapidly and radically changing our lives, which will improve our health and longevity.
Aubrey de Grey, from Nov 28, 2017.
Virtual Futures presents Dr. Aubrey de Grey who claims to have drawn a roadmap to defeat biological aging and proposes that that the first human beings who will live to 1,000 years old have already been born.
Dr Aubrey de Grey is the Chief Scientific Officer and Co-founder of the SENS Research Foundation, a charity that researches the application of regenerative medicine to age-related disease, with the intent of repairing underlying damage to the body’s tissues, cells, and molecules. Their goal is to help build the industry that will cure the diseases of aging.
In conversation with Luke Robert Mason (Director, Virtual Futures).
–
Aubrey de Grey of the SENS Research Foundation took a few hours from his packed schedule yesterday to answer questions from the community at /r/futurology. It is a pity that we can’t get a full day of his time at some point — clearly there are way too many interested folk with questions and not enough hours to answer more than half of them. It is a sign of progress, I hope, that ever more people recognize that the SENS approach to the development of rejuvenation therapies is promising, and understand enough of the science to ask intelligent questions about the details.
SENS is simple enough to explain at the high level: identify the cell and tissue damage that (a) appears in old tissues but not in young tissues, and (b) is caused by the normal operation of metabolism, not by some other form of damage. The resulting short list includes the causes of aging. It may include some other things as well, that in the end turn out not to need fixing, but why take the chance? In modern biotechnology and life science research, it is faster and cheaper to develop a repair therapy and see what happens than it is to painstakingly figure out how everything fits together.
When de Grey first evaluated the field of aging research, back before the turn of the century, he found that the causes of aging by the above definition were largely known, with a good deal of evidence in support of each one. Yet next to no-one was working on fixing them. Since then, he has campaigned tirelessly to build organisations, assemble allies, raise funding, and persuade researchers, and all of that to ensure that the scientific and biotechnology communities do in fact move ahead with a repair-based approach to building functional rejuvenation therapies. It has been surprisingly hard work, given a research community that was hostile towards the idea of treating aging as a medical condition versus merely observing it, and a public at large who seem disinterested in living longer in good health.