Why our metabolism slows down as we age and the diseases it contributes to.
Researchers discover reasons for our age-related metabolic slowdown and show that metabolic changes lead to disease and aging.


The benefits of rejuvenation biotechnology to end age-related diseases could go beyond just the individual.
As I wrote in a different article, rejuvenation biotechnology promises a range of benefits for individuals. Lest anyone thinks that’s all rejuvenation has to offer, I reckon it’s worth discussing other ways that this technology would benefit larger groups of people—namely, your friends and family. If you are rejuvenated, that’s all good for you, but is there anything good coming out of it for your dear ones? Oh, yes.
Two burdens relieved with a single shot
The ill health of old age is a formidable sword of Damocles looming over us all, and when it falls down, it typically does not hit just us; the elderly are certainly the primary victims, but their family are collateral casualties. When people lose their health and independence to aging, their families have to go through the pain of seeing their loved ones becoming more and more fragile, sick, dependent, perhaps even demented. Adding insult to injury, the troubles caused by aging don’t stop here, because a sick and dependent person needs looking after. Thus, the family of an elderly person needs to step in themselves to take care of their relative; if this is not possible, a nursing home is likely going to be the only option left.

Izpisúa, Blasco, De Grey, and Magalhães meet in Madrid at the end of summer at the first “International Longevity and Cryopreservation Summit.” The conference lasts two days and is held in the CSIC, attracting prominent scientists, futurists and freaks, as conference organizer and Vidaplus President Txetxu Mazuelas, refers to them. The scientific world and the futuristic world inevitably clash. One of the most heated debates is on the cryopreservation of human beings – a kind of plan B that puts humans on ice while they work out the secret to eternal life.
Could we live to 140? 1,000? Is there a limit? Scientific research into extending the human lifespan is being backed by Silicon Valley giants such as Google and Facebook.

Speaking of concerns, I’m a bit concerned that the discussion about what might go wrong or how to prevent this or that hypothetical problem might draw attention away from another, possibly even more important question: Why do we strive to make rejuvenation a reality? There’s not much point in doing something if it doesn’t yield any benefits, especially if that something requires as much hard work as this cause does; so, what are the expected benefits of rejuvenation?
The benefits are many; some are obvious, and some are less so. The ones I’ll discuss in this article are the ones I see as obvious—tangible, immediate benefits for the people undergoing rejuvenation.

Really excited to announce my interview in Allure Magazine, one of the biggest women’s mags out there. This is also in print as their November “Science” Issue with 1.2 million distribution: I believe it’s the first time #transhumanism has been in their mag and shows how widespread the movement is getting. 2 other longevity interviews as well in story:
If you’ve ever thought it might be nice to live forever, you’re in impressive company. From moon-shot projects to billionaire-funded research, three experts share vastly different views on the future of what it means to be human.
Laura Carstensen is the director of the Center of Longevity at Stanford University and the author of A Long Bright Future: Happiness, Health, and Financial Security in an Age of Increased Longevity (PublicAffairs).
You May Also Like

I often hear the claim that death is a statistical certainty. This is my mathematical take at why this is not true, assuming the defeat of ageing, and no, being immortal is not required.
If a fully rejuvenated person was hit by a train at full speed, I can promise you they would stand the same pathetically low chances of ever being reassembled into a single, barely functional piece as any non-rejuvenated person of any age. Keeping that in mind, if anyone tried to sell me rejuvenation as ‘immortality’, rest assured I would demand to see the manager right away.
On a different yet unexpectedly related note, if I had a nickel for every time I heard or read something along the lines of ‘death is inevitable because probability’, I could donate so much money to LEAF the IRS would start thinking they’re a bit too well off for a charity.
Oh, and with the rest of the money, I could buy LinkedIn and pay someone to finally give it a user interface you can look at without your eyes bleeding.

Whenever the topic of increasing human lifespan is discussed the concern is sometimes raised that a longer life would mean a life spent frail and decrepit. This is sometimes known as the Tithonus error and shows a fundamental misunderstanding of the aims of rejuvenation biotechnology. The concern is based on the ancient Greek myth of Tithonus which might be thought of as a cautionary tale warning seekers of an eternal life of its alleged inherent dangers.
We want to hear your story too!
https://www.leafscience.org/longevity-month-2017-tell-us-your-story/
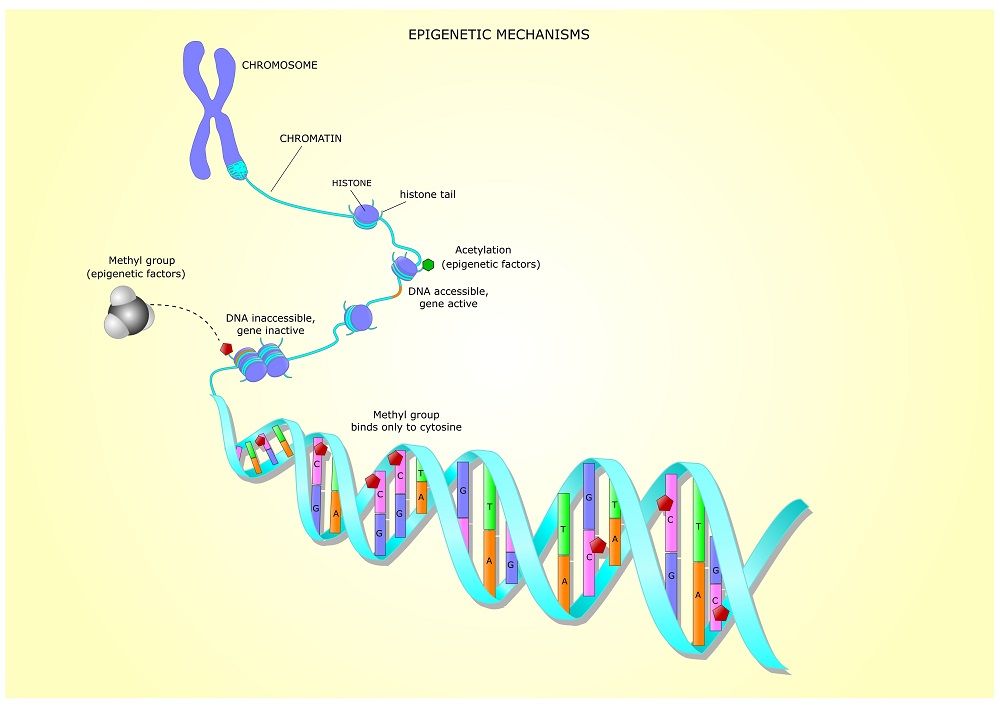
This is the second part of our ongoing series of articles that discuss the Hallmarks of Aging. Published in 2013, the paper divides aging into a number of distinct categories (“hallmarks”) of damage to explain how the aging process works and how it causes age-related diseases[1].
Today, we will be looking at one of the primary hallmarks, epigenetic alterations.